Molecular Mechanisms of Airway Hyperresponsiveness in a Murine Model of Steroid-Resistant Airway Inflammation
- PMID: 26729801
- PMCID: PMC4724491
- DOI: 10.4049/jimmunol.1501531
Molecular Mechanisms of Airway Hyperresponsiveness in a Murine Model of Steroid-Resistant Airway Inflammation
Abstract
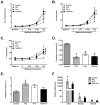
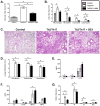
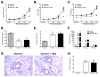
IL-13 and IL-17A, produced mainly by Th2 and Th17 cells, respectively, have an influential role in asthma pathogenesis. We examined the role of IL-13 and IL-17A in mediating airway hyperresponsiveness (AHR), lung inflammation, and mucus metaplasia in a dual Th2/Th17 model of asthma. IL-13 and/or IL-17A were neutralized using mAbs. Th2/Th17 adoptive transfer induced a mixed asthma phenotype characterized by elevated eosinophilia and neutrophilia, tissue inflammation, mucus metaplasia, and AHR that were partially reversible with steroid treatment. Pulmonary inflammation and quasi-static lung compliance were largely unaffected by neutralization of IL-13 and/or IL-17A. However, neutralization of IL-13 alone or in combination with IL-17A significantly attenuated AHR and mucus metaplasia. Further, STAT6 activation was attenuated following IL-13 and IL-13/IL-17A Ab treatment. We next assessed the role of STAT6 in Th2/Th17-mediated allergic airway disease using STAT6(-/-) mice. STAT6(-/-) mice adoptively transferred with Th2/Th17 cells had decreased AHR compared with controls. These data suggest that IL-13 drives AHR and mucus metaplasia in a STAT6-dependent manner, without directly contributing to airway or tissue inflammation. IL-17A independently contributes to AHR, but it only partially mediates inflammation and mucus metaplasia in a mixed Th2/Th17 model of steroid-resistant asthma.
Copyright © 2016 by The American Association of Immunologists, Inc.
Figures
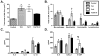
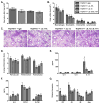
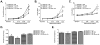

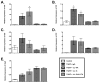
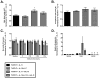
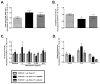
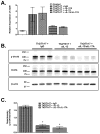
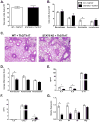

Comment in
-
Do insights from mice imply that combined Th2 and Th17 therapies would benefit select severe asthma patients?Ann Transl Med. 2016 Dec;4(24):505. doi: 10.21037/atm.2016.11.79. Ann Transl Med. 2016. PMID: 28149867 Free PMC article. No abstract available.
Similar articles
-
IL-17A induces signal transducers and activators of transcription-6-independent airway mucous cell metaplasia.Am J Respir Cell Mol Biol. 2013 Jun;48(6):711-6. doi: 10.1165/rcmb.2013-0017OC. Am J Respir Cell Mol Biol. 2013. PMID: 23392574 Free PMC article.
-
The endogenous Th17 response in NO2-promoted allergic airway disease is dispensable for airway hyperresponsiveness and distinct from Th17 adoptive transfer.PLoS One. 2013 Sep 19;8(9):e74730. doi: 10.1371/journal.pone.0074730. eCollection 2013. PLoS One. 2013. PMID: 24069338 Free PMC article.
-
Contribution of IL-18-induced innate T cell activation to airway inflammation with mucus hypersecretion and airway hyperresponsiveness.Int Immunol. 2006 Jun;18(6):847-55. doi: 10.1093/intimm/dxl021. Epub 2006 Apr 12. Int Immunol. 2006. PMID: 16611648
-
Allergic airway inflammation: key players beyond the Th2 cell pathway.Immunol Rev. 2017 Jul;278(1):145-161. doi: 10.1111/imr.12540. Immunol Rev. 2017. PMID: 28658544 Review.
-
Th17/IL-17 Axis Regulated by Airway Microbes Get Involved in the Development of Asthma.Curr Allergy Asthma Rep. 2020 Mar 14;20(4):11. doi: 10.1007/s11882-020-00903-x. Curr Allergy Asthma Rep. 2020. PMID: 32172346 Review.
Cited by
-
Effect of Anti-IL17 Antibody Treatment Alone and in Combination With Rho-Kinase Inhibitor in a Murine Model of Asthma.Front Physiol. 2018 Sep 5;9:1183. doi: 10.3389/fphys.2018.01183. eCollection 2018. Front Physiol. 2018. PMID: 30233389 Free PMC article.
-
Pyroptosis and respiratory diseases: A review of current knowledge.Front Immunol. 2022 Sep 30;13:920464. doi: 10.3389/fimmu.2022.920464. eCollection 2022. Front Immunol. 2022. PMID: 36248872 Free PMC article. Review.
-
MBD2 as a Potential Novel Biomarker for Identifying Severe Asthma With Different Endotypes.Front Med (Lausanne). 2021 Oct 5;8:693605. doi: 10.3389/fmed.2021.693605. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34692717 Free PMC article.
-
Co-Stimulation with TWEAK and TGF-β1 Induces Steroid-Insensitive TSLP and CCL5 Production in BEAS-2B Human Bronchial Epithelial Cells.Int J Mol Sci. 2024 Oct 29;25(21):11625. doi: 10.3390/ijms252111625. Int J Mol Sci. 2024. PMID: 39519176 Free PMC article.
-
Distinct spatial and temporal roles for Th1, Th2, and Th17 cells in asthma.Front Immunol. 2022 Aug 12;13:974066. doi: 10.3389/fimmu.2022.974066. eCollection 2022. Front Immunol. 2022. PMID: 36032162 Free PMC article. Review.
References
-
- Wenzel S. Severe asthma: from characteristics to phenotypes to endotypes. Clin Exp Allergy. 2012;42:650–658. - PubMed
-
- Xie M, Wenzel SE. A global perspective in asthma: from phenotype to endotype. Chinese medical journal. 2013;126:166–174. - PubMed
-
- Hellings PW, Kasran A, Liu Z, Vandekerckhove P, Wuyts A, Overbergh L, Mathieu C, Ceuppens JL. Interleukin-17 orchestrates the granulocyte influx into airways after allergen inhalation in a mouse model of allergic asthma. Am J Respir Cell Mol Biol. 2003;28:42–50. - PubMed
-
- Aujla SJ, Alcorn JF. T(H)17 cells in asthma and inflammation. Biochim Biophys Acta. 2011;1810:1066–1079. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

