The Immunodominance Change and Protection of CD4+ T-Cell Responses Elicited by an Envelope Protein Domain III-Based Tetravalent Dengue Vaccine in Mice
- PMID: 26714037
- PMCID: PMC4695087
- DOI: 10.1371/journal.pone.0145717
The Immunodominance Change and Protection of CD4+ T-Cell Responses Elicited by an Envelope Protein Domain III-Based Tetravalent Dengue Vaccine in Mice
Abstract
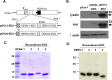
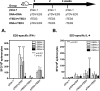
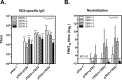
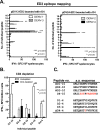
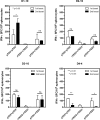
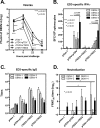
Dengue is the leading cause of mosquito-borne viral infections and no vaccine is available now. Envelope protein domain III (ED3) is the major target for the binding of dengue virus neutralizing antibodies; however, the ED3-specifc T-cell response is less well understood. To investigate the T-cell responses to four serotypes of dengue virus (DENV-1 to 4), we immunized mice using either a tetravalent ED3-based DNA or protein vaccine, or combined both as a DNA prime-protein boost strategy (prime-boost). A significant serotype-dependent IFN-γ or IL-4 response was observed in mice immunized with either the DNA or protein vaccine. The IFN-γ response was dominant to DENV-1 to 3, whereas the IL-4 response was dominant to DENV-4. Although the similar IgG titers for the four serotypes were observed in mice immunized with the tetravalent vaccines, the neutralizing antibody titers varied and followed the order of 2 = 3>1>4. Interestingly, the lower IFN-γ response to DENV-4 is attributable to the immunodominance change between two CD4+ T-cell epitopes; one T-cell epitope located at E349-363 of DENV-1 to 3 was more immunogenic than the DENV-4 epitope E313-327. Despite DENV-4 specific IFN-γ responses were suppressed by immunodominance change, either DENV-4-specific IFN-γ or neutralizing antibody responses were still recalled after DENV-4 challenge and contributed to virus clearance. Immunization with the prime-boost elicited both IFN-γ and neutralizing antibody responses and provided better protection than either DNA or protein immunization. Our findings shed light on how ED3-based tetravalent dengue vaccines sharpen host CD4 T-cell responses and contribute to protection against dengue virus.
Conflict of interest statement
Figures
Similar articles
-
Immunodomination of Serotype-Specific CD4+ T-Cell Epitopes Contributed to the Biased Immune Responses Induced by a Tetravalent Measles-Vectored Dengue Vaccine.Front Immunol. 2020 Mar 31;11:546. doi: 10.3389/fimmu.2020.00546. eCollection 2020. Front Immunol. 2020. PMID: 32300346 Free PMC article.
-
An Envelope-Modified Tetravalent Dengue Virus-Like-Particle Vaccine Has Implications for Flavivirus Vaccine Design.J Virol. 2017 Nov 14;91(23):e01181-17. doi: 10.1128/JVI.01181-17. Print 2017 Dec 1. J Virol. 2017. PMID: 28956764 Free PMC article.
-
Dengue virus envelope domain III immunization elicits predominantly cross-reactive, poorly neutralizing antibodies localized to the AB loop: implications for dengue vaccine design.J Gen Virol. 2013 Oct;94(Pt 10):2191-2201. doi: 10.1099/vir.0.055178-0. Epub 2013 Jul 12. J Gen Virol. 2013. PMID: 23851440
-
Identification and selection of immunodominant B and T cell epitopes for dengue multi-epitope-based vaccine.Med Microbiol Immunol. 2021 Feb;210(1):1-11. doi: 10.1007/s00430-021-00700-x. Epub 2021 Jan 30. Med Microbiol Immunol. 2021. PMID: 33515283 Review.
-
Antibody-Dependent Enhancement: A Challenge for Developing a Safe Dengue Vaccine.Front Cell Infect Microbiol. 2020 Oct 22;10:572681. doi: 10.3389/fcimb.2020.572681. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33194810 Free PMC article. Review.
Cited by
-
Solubility Controlling Peptide Tags of Opposite Charges Generate a Bivalent Immune Response Against Dengue ED3 Serotypes 3 and 4.Front Immunol. 2021 Jun 11;12:671590. doi: 10.3389/fimmu.2021.671590. eCollection 2021. Front Immunol. 2021. PMID: 34177912 Free PMC article.
-
An Overview of Current Uses and Future Opportunities for Computer-Assisted Design of Vaccines for Neglected Tropical Diseases.Adv Appl Bioinform Chem. 2021 Feb 15;14:25-47. doi: 10.2147/AABC.S258759. eCollection 2021. Adv Appl Bioinform Chem. 2021. PMID: 33623396 Free PMC article. Review.
-
T Cell Responses Induced by DNA Vaccines Based on the DENV2 E and NS1 Proteins in Mice: Importance in Protection and Immunodominant Epitope Identification.Front Immunol. 2019 Jul 3;10:1522. doi: 10.3389/fimmu.2019.01522. eCollection 2019. Front Immunol. 2019. PMID: 31333657 Free PMC article.
-
Combination of E- and NS1-Derived DNA Vaccines: The Immune Response and Protection Elicited in Mice against DENV2.Viruses. 2022 Jun 30;14(7):1452. doi: 10.3390/v14071452. Viruses. 2022. PMID: 35891431 Free PMC article.
-
Immunodomination of Serotype-Specific CD4+ T-Cell Epitopes Contributed to the Biased Immune Responses Induced by a Tetravalent Measles-Vectored Dengue Vaccine.Front Immunol. 2020 Mar 31;11:546. doi: 10.3389/fimmu.2020.00546. eCollection 2020. Front Immunol. 2020. PMID: 32300346 Free PMC article.
References
-
- Thomas SJ, Endy TP. Vaccines for the prevention of dengue: development update. Hum Vaccin. 2011;7(6):674–84. Epub 2011/04/22. 14985 [pii]. . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

