SerpinB1 Promotes Pancreatic β Cell Proliferation
- PMID: 26701651
- PMCID: PMC4715773
- DOI: 10.1016/j.cmet.2015.12.001
SerpinB1 Promotes Pancreatic β Cell Proliferation
Abstract
Although compensatory islet hyperplasia in response to insulin resistance is a recognized feature in diabetes, the factor(s) that promote β cell proliferation have been elusive. We previously reported that the liver is a source for such factors in the liver insulin receptor knockout (LIRKO) mouse, an insulin resistance model that manifests islet hyperplasia. Using proteomics we show that serpinB1, a protease inhibitor, which is abundant in the hepatocyte secretome and sera derived from LIRKO mice, is the liver-derived secretory protein that regulates β cell proliferation in humans, mice, and zebrafish. Small-molecule compounds, that partially mimic serpinB1 effects of inhibiting elastase activity, enhanced proliferation of β cells, and mice lacking serpinB1 exhibit attenuated β cell compensation in response to insulin resistance. Finally, SerpinB1 treatment of islets modulated proteins in growth/survival pathways. Together, these data implicate serpinB1 as an endogenous protein that can potentially be harnessed to enhance functional β cell mass in patients with diabetes.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures

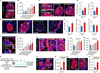
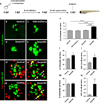
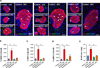


Comment in
-
Dramatis Personae in β-Cell Mass Regulation: Enter SerpinB1.Cell Metab. 2016 Jan 12;23(1):8-10. doi: 10.1016/j.cmet.2015.12.011. Cell Metab. 2016. PMID: 26771113
Similar articles
-
Forkhead box protein O1 (FoxO1) regulates hepatic serine protease inhibitor B1 (serpinB1) expression in a non-cell-autonomous fashion.J Biol Chem. 2019 Jan 18;294(3):1059-1069. doi: 10.1074/jbc.RA118.006031. Epub 2018 Nov 20. J Biol Chem. 2019. PMID: 30459233 Free PMC article.
-
Insulin receptors in beta-cells are critical for islet compensatory growth response to insulin resistance.Proc Natl Acad Sci U S A. 2007 May 22;104(21):8977-82. doi: 10.1073/pnas.0608703104. Epub 2007 Apr 6. Proc Natl Acad Sci U S A. 2007. PMID: 17416680 Free PMC article.
-
Liver-derived systemic factors drive β cell hyperplasia in insulin-resistant states.Cell Rep. 2013 Feb 21;3(2):401-10. doi: 10.1016/j.celrep.2013.01.007. Epub 2013 Jan 31. Cell Rep. 2013. PMID: 23375376 Free PMC article.
-
Modulation of β-cell function: a translational journey from the bench to the bedside.Diabetes Obes Metab. 2012 Oct;14 Suppl 3:152-60. doi: 10.1111/j.1463-1326.2012.01647.x. Diabetes Obes Metab. 2012. PMID: 22928576 Review.
-
Receptors for insulin and insulin-like growth factor-1 and insulin receptor substrate-1 mediate pathways that regulate islet function.Biochem Soc Trans. 2002 Apr;30(2):317-22. doi: 10.1042/bst0300317. Biochem Soc Trans. 2002. PMID: 12023872 Review.
Cited by
-
Redox regulation of m6A methyltransferase METTL3 in β-cells controls the innate immune response in type 1 diabetes.Nat Cell Biol. 2024 Mar;26(3):421-437. doi: 10.1038/s41556-024-01368-0. Epub 2024 Feb 26. Nat Cell Biol. 2024. PMID: 38409327 Free PMC article.
-
Single-Cell RNA Sequencing Reveals a Role for Reactive Oxygen Species and Peroxiredoxins in Fatty Acid-Induced Rat β-Cell Proliferation.Diabetes. 2023 Jan 1;72(1):45-58. doi: 10.2337/db22-0121. Diabetes. 2023. PMID: 36191509 Free PMC article.
-
HB-EGF Signaling Is Required for Glucose-Induced Pancreatic β-Cell Proliferation in Rats.Diabetes. 2020 Mar;69(3):369-380. doi: 10.2337/db19-0643. Epub 2019 Dec 27. Diabetes. 2020. PMID: 31882563 Free PMC article.
-
β-Cell senescence in the pathogenesis of type 2 diabetes.J Diabetes Investig. 2020 Mar;11(2):284-286. doi: 10.1111/jdi.13162. Epub 2019 Nov 19. J Diabetes Investig. 2020. PMID: 31618527 Free PMC article.
-
Toll-like receptors TLR2 and TLR4 block the replication of pancreatic β cells in diet-induced obesity.Nat Immunol. 2019 Jun;20(6):677-686. doi: 10.1038/s41590-019-0396-z. Epub 2019 May 20. Nat Immunol. 2019. PMID: 31110312 Free PMC article.
References
-
- Amrein PC, Stossel TP. Prevention of degradation of human polymorphonuclear leukocyte proteins by diisopropylfluorophosphate. Blood. 1980;56:442–447. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P41 GM103493/GM/NIGMS NIH HHS/United States
- R21 AI103407/AI/NIAID NIH HHS/United States
- R00 DK090210/DK/NIDDK NIH HHS/United States
- K99 DK090210/DK/NIDDK NIH HHS/United States
- R01 DK 074795/DK/NIDDK NIH HHS/United States
- R01 HL066548/HL/NHLBI NIH HHS/United States
- R01 DK055523/DK/NIDDK NIH HHS/United States
- P30 DK036836/DK/NIDDK NIH HHS/United States
- R01 DK074795/DK/NIDDK NIH HHS/United States
- UC4 DK104167/DK/NIDDK NIH HHS/United States
- R01 DK103215/DK/NIDDK NIH HHS/United States
- R01 DK67536/DK/NIDDK NIH HHS/United States
- R01 DK067536/DK/NIDDK NIH HHS/United States
- R01 DK55523/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

