The Regulation of the AdcR Regulon in Streptococcus pneumoniae Depends Both on Zn(2+)- and Ni(2+)-Availability
- PMID: 26697415
- PMCID: PMC4672087
- DOI: 10.3389/fcimb.2015.00091
The Regulation of the AdcR Regulon in Streptococcus pneumoniae Depends Both on Zn(2+)- and Ni(2+)-Availability
Abstract
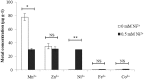
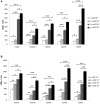
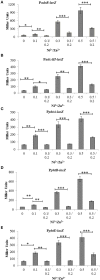
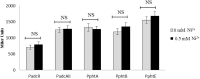
By using a transcriptomic approach, we have elucidated the effect of Ni(2+) on the global gene expression of S. pneumoniae D39 by identifying several differentially expressed genes/operons in the presence of a high extracellular concentration of Ni(2+). The genes belonging to the AdcR regulon (adcRCBA, adcAII-phtD, phtA, phtB, and phtE) and the PsaR regulon (pcpA, prtA, and psaBCA) were highly upregulated in the presence of Ni(2+). We have further studied the role of Ni(2+) in the regulation of the AdcR regulon by using ICP-MS analysis, electrophoretic mobility shift assays and transcriptional lacZ-reporter studies, and demonstrate that Ni(2+) is directly involved in the derepression of the AdcR regulon via the Zn(2+)-dependent repressor AdcR, and has an opposite effect on the expression of the AdcR regulon compared to Zn(2+).
Keywords: AdcR; AdcR regulon; Pht family proteins; PsaR regulon; metal homeostasis; nickel; pneumococcus; zinc.
Figures

Similar articles
-
Transcriptional response of Streptococcus pneumoniae to Zn2+) limitation and the repressor/activator function of AdcR.Metallomics. 2011 Jun;3(6):609-18. doi: 10.1039/c1mt00030f. Epub 2011 May 21. Metallomics. 2011. PMID: 21603707
-
Ni2+-Dependent and PsaR-Mediated Regulation of the Virulence Genes pcpA, psaBCA, and prtA in Streptococcus pneumoniae.PLoS One. 2015 Nov 12;10(11):e0142839. doi: 10.1371/journal.pone.0142839. eCollection 2015. PLoS One. 2015. PMID: 26562538 Free PMC article.
-
Opposite effects of Mn2+ and Zn2+ on PsaR-mediated expression of the virulence genes pcpA, prtA, and psaBCA of Streptococcus pneumoniae.J Bacteriol. 2008 Aug;190(15):5382-93. doi: 10.1128/JB.00307-08. Epub 2008 May 30. J Bacteriol. 2008. PMID: 18515418 Free PMC article.
-
The metalloregulatory zinc site in Streptococcus pneumoniae AdcR, a zinc-activated MarR family repressor.J Mol Biol. 2010 Oct 22;403(2):197-216. doi: 10.1016/j.jmb.2010.08.030. Epub 2010 Sep 8. J Mol Biol. 2010. PMID: 20804771 Free PMC article.
-
The Adc regulon mediates zinc homeostasis in Streptococcus mutans.Mol Oral Microbiol. 2021 Oct;36(5):278-290. doi: 10.1111/omi.12350. Epub 2021 Aug 13. Mol Oral Microbiol. 2021. PMID: 34351080
Cited by
-
PmtA functions as a ferrous iron and cobalt efflux pump in Streptococcus suis.Emerg Microbes Infect. 2019;8(1):1254-1264. doi: 10.1080/22221751.2019.1660233. Emerg Microbes Infect. 2019. PMID: 31469035 Free PMC article.
-
Zinc-Dependent Transcriptional Regulation in Paracoccus denitrificans.Front Microbiol. 2017 Apr 11;8:569. doi: 10.3389/fmicb.2017.00569. eCollection 2017. Front Microbiol. 2017. PMID: 28443074 Free PMC article.
-
Streptococcus pneumoniae metal homeostasis alters cellular metabolism.Metallomics. 2020 Sep 23;12(9):1416-1427. doi: 10.1039/d0mt00118j. Metallomics. 2020. PMID: 32676626 Free PMC article.
-
The adcA and lmb Genes Play an Important Role in Drug Resistance and Full Virulence of Streptococcus suis.Microbiol Spectr. 2023 Jun 15;11(3):e0433722. doi: 10.1128/spectrum.04337-22. Epub 2023 May 22. Microbiol Spectr. 2023. PMID: 37212676 Free PMC article.
-
Adaptation to zinc restriction in Streptococcus agalactiae: role of the ribosomal protein and zinc-importers regulated by AdcR.mSphere. 2024 Nov 21;9(11):e0061424. doi: 10.1128/msphere.00614-24. Epub 2024 Oct 31. mSphere. 2024. PMID: 39480081 Free PMC article.
References
-
- Alimonti A., Bocca B., Mannella E., Petrucci F., Zennaro F., Cotichini R., et al. . (2005). Assessment of reference values for selected elements in a healthy urban population. Ann. Ist. Super. Sanità 41, 181–187. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

