The Epstein-Barr Virus BART miRNA Cluster of the M81 Strain Modulates Multiple Functions in Primary B Cells
- PMID: 26694854
- PMCID: PMC4691206
- DOI: 10.1371/journal.ppat.1005344
The Epstein-Barr Virus BART miRNA Cluster of the M81 Strain Modulates Multiple Functions in Primary B Cells
Abstract
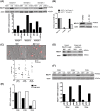
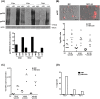
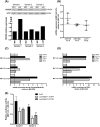
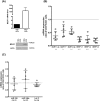
The Epstein-Barr virus (EBV) is a B lymphotropic virus that infects the majority of the human population. All EBV strains transform B lymphocytes, but some strains, such as M81, also induce spontaneous virus replication. EBV encodes 22 microRNAs (miRNAs) that form a cluster within the BART region of the virus and have been previously been found to stimulate tumor cell growth. Here we describe their functions in B cells infected by M81. We found that the BART miRNAs are downregulated in replicating cells, and that exposure of B cells in vitro or in vivo in humanized mice to a BART miRNA knockout virus resulted in an increased proportion of spontaneously replicating cells, relative to wild type virus. The BART miRNAs subcluster 1, and to a lesser extent subcluster 2, prevented expression of BZLF1, the key protein for initiation of lytic replication. Thus, multiple BART miRNAs cooperate to repress lytic replication. The BART miRNAs also downregulated pro- and anti-apoptotic mediators such as caspase 3 and LMP1, and their deletion did not sensitize B-cells to apoptosis. To the contrary, the majority of humanized mice infected with the BART miRNA knockout mutant developed tumors more rapidly, probably due to enhanced LMP1 expression, although deletion of the BART miRNAs did not modify the virus transforming abilities in vitro. This ability to slow cell growth could be confirmed in non-humanized immunocompromized mice. Injection of resting B cells exposed to a virus that lacks the BART miRNAs resulted in accelerated tumor growth, relative to wild type controls. Therefore, we found that the M81 BART miRNAs do not enhance B-cell tumorigenesis but rather repress it. The repressive effects of the BART miRNAs on potentially pathogenic viral functions in infected B cells are likely to facilitate long-term persistence of the virus in the infected host.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
NF-κB Signaling Regulates Expression of Epstein-Barr Virus BART MicroRNAs and Long Noncoding RNAs in Nasopharyngeal Carcinoma.J Virol. 2016 Jun 24;90(14):6475-88. doi: 10.1128/JVI.00613-16. Print 2016 Jul 15. J Virol. 2016. PMID: 27147748 Free PMC article.
-
The biological properties of different Epstein-Barr virus strains explain their association with various types of cancers.Oncotarget. 2017 Feb 7;8(6):10238-10254. doi: 10.18632/oncotarget.14380. Oncotarget. 2017. PMID: 28052012 Free PMC article.
-
A viral microRNA cluster strongly potentiates the transforming properties of a human herpesvirus.PLoS Pathog. 2011 Feb;7(2):e1001294. doi: 10.1371/journal.ppat.1001294. Epub 2011 Feb 17. PLoS Pathog. 2011. PMID: 21379335 Free PMC article.
-
Role of Viral and Host microRNAs in Immune Regulation of Epstein-Barr Virus-Associated Diseases.Front Immunol. 2020 Mar 3;11:367. doi: 10.3389/fimmu.2020.00367. eCollection 2020. Front Immunol. 2020. PMID: 32194570 Free PMC article. Review.
-
Regulation and dysregulation of Epstein-Barr virus latency: implications for the development of autoimmune diseases.Autoimmunity. 2008 May;41(4):298-328. doi: 10.1080/08916930802024772. Autoimmunity. 2008. PMID: 18432410 Review.
Cited by
-
Viral non-coding RNAs: Stealth strategies in the tug-of-war between humans and herpesviruses.Semin Cell Dev Biol. 2021 Mar;111:135-147. doi: 10.1016/j.semcdb.2020.06.015. Epub 2020 Jul 3. Semin Cell Dev Biol. 2021. PMID: 32631785 Free PMC article. Review.
-
Identification and Cloning of a New Western Epstein-Barr Virus Strain That Efficiently Replicates in Primary B Cells.J Virol. 2020 May 4;94(10):e01918-19. doi: 10.1128/JVI.01918-19. Print 2020 May 4. J Virol. 2020. PMID: 32102884 Free PMC article.
-
Epstein-Barr Virus as a Promising Immunotherapeutic Target for Nasopharyngeal Carcinoma Treatment.J Pathog. 2017;2017:7349268. doi: 10.1155/2017/7349268. Epub 2017 Dec 31. J Pathog. 2017. PMID: 29464124 Free PMC article. Review.
-
Epstein-Barr virus NK and T cell lymphoproliferative disease: report of a 2018 international meeting.Leuk Lymphoma. 2020 Apr;61(4):808-819. doi: 10.1080/10428194.2019.1699080. Epub 2019 Dec 13. Leuk Lymphoma. 2020. PMID: 31833428 Free PMC article. Review.
-
The Epstein-Barr virus noncoding RNA EBER2 transactivates the UCHL1 deubiquitinase to accelerate cell growth.Proc Natl Acad Sci U S A. 2021 Oct 26;118(43):e2115508118. doi: 10.1073/pnas.2115508118. Proc Natl Acad Sci U S A. 2021. PMID: 34686609 Free PMC article.
References
-
- Kieff ED, Rickinson AB (2007) Epstein-Barr Virus and its replication In: Knipe DM HP, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE, editor. Field Virology. Philadelphia: Lippincott Williams & Wilkins; pp. 2603–2654.
-
- Rickinson AB, Kieff E (2007) Epstein-Barr virus In: Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA et al., editors. Fields Virology. 5th Edition ed. Philadelphia: Lippincott Williams & Wilkins; pp. 2655–2700.
-
- Pfeffer S, Zavolan M, Grasser FA, Chien M, Russo JJ, et al. (2004) Identification of virus-encoded microRNAs. Science 304: 734–736. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

