Combining mesenchymal stem cell sheets with platelet-rich plasma gel/calcium phosphate particles: a novel strategy to promote bone regeneration
- PMID: 26689714
- PMCID: PMC4687276
- DOI: 10.1186/s13287-015-0256-1
Combining mesenchymal stem cell sheets with platelet-rich plasma gel/calcium phosphate particles: a novel strategy to promote bone regeneration
Abstract
Background: Promotion of bone regeneration is important for successful repair of bony defects. This study aimed to investigate whether combining bone marrow-derived mesenchymal stem cell (BMSC) sheets with platelet-rich plasma (PRP) gel/calcium phosphate particles could promote bone formation in the femoral bone defects of rats.
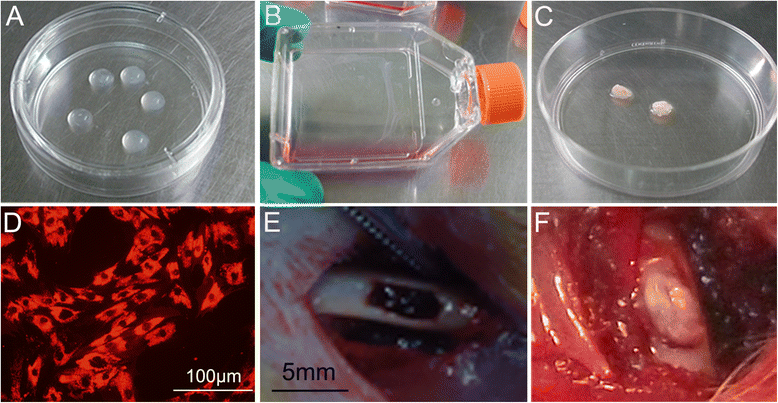
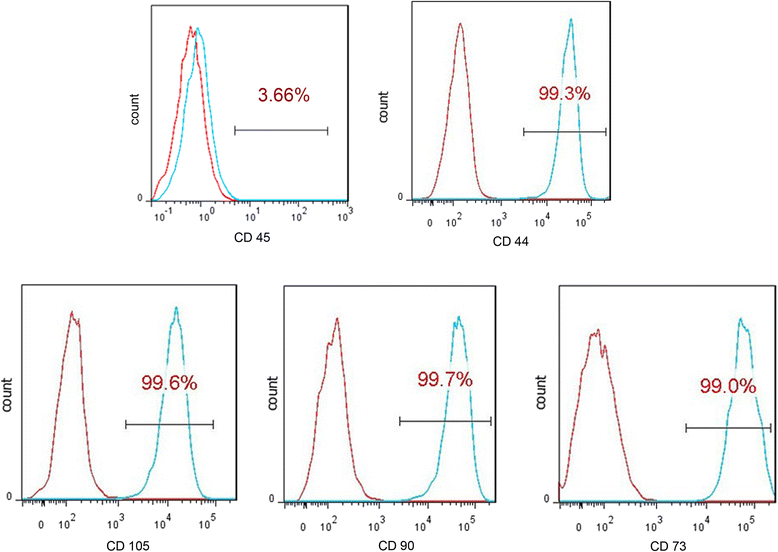
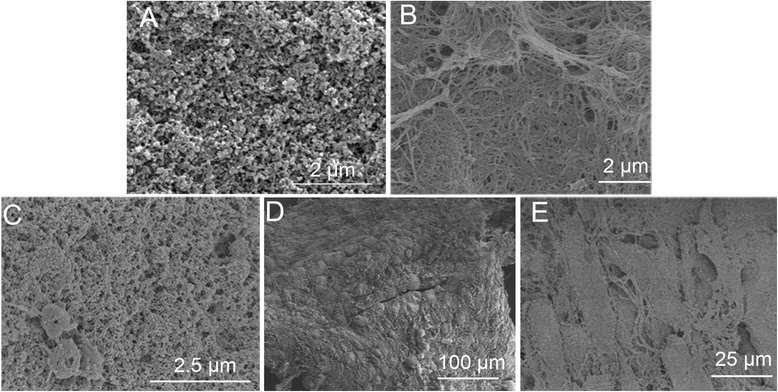
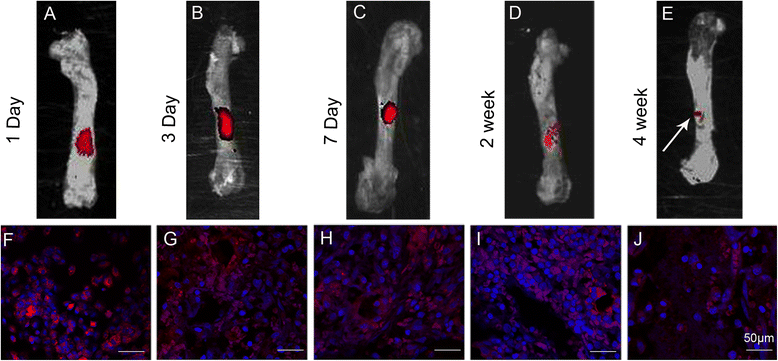
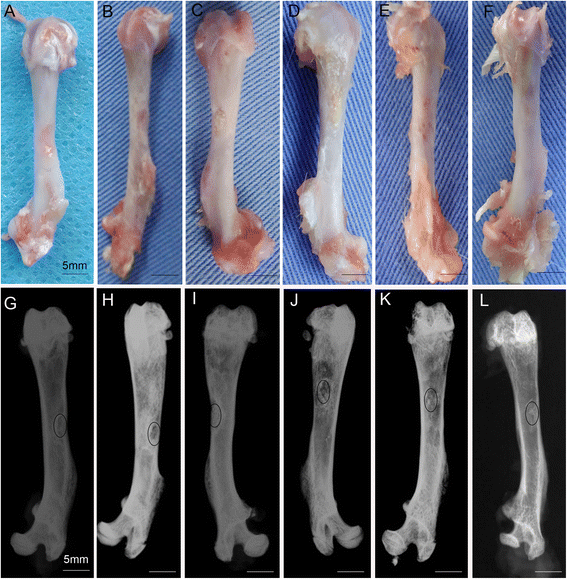
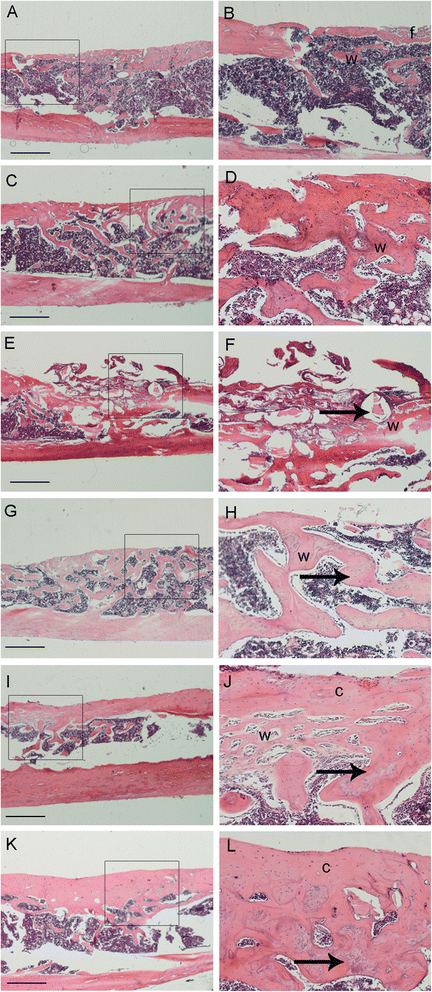
Methods: The proliferation and differentiation of BMSCs or BMSC sheets cultured with calcium phosphate particles and/or PRP were investigated in in vitro. In vivo, 36 2.5 × 5 mm bone defects were randomly divided into groups and treated with either BMSCs/PRP gel, calcium phosphate particles, PRP gel/calcium phosphate particles, a BMSC sheet/calcium phosphate particles, a BMSC sheet/PRP gel/calcium phosphate particles, or were left untreated (n = 6/group). A further 15 bone defects were treated with chloromethyl-benzamidodialkylcarbocyanine (CM-Dil)-labelled BMSC sheet/PRP gel/calcium phosphate particles and observed using a small animal in vivo fluorescence imaging system to trace the implanted BMSCs at 1 day, 3 days, 7 days, 2 weeks, and 4 weeks after surgery.
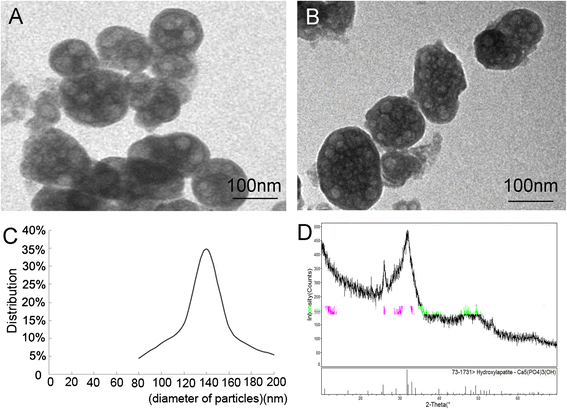
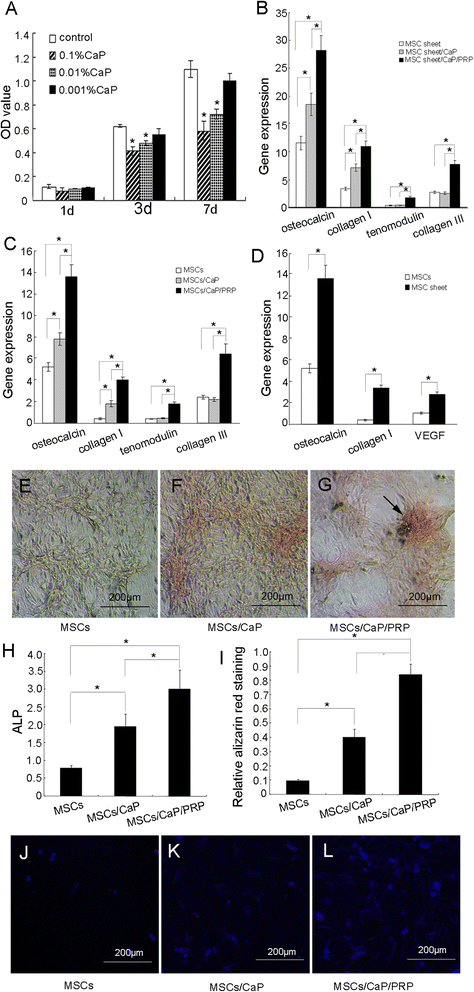
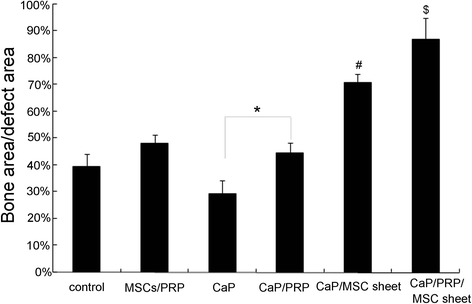
Results: The expression of collagen type I and osteocalcin genes of BMSCs or BMSC sheets treated with PRP and calcium phosphate particles was significantly higher than that of BMSCs or BMSC sheets treated with calcium phosphate particles or the controls (P <0.05). PRP can promote gene expression of collagen III and tenomodulin by BMSCs and in BMSC sheets. The VEGF, collagen I and osteocalcin gene expression levels were higher in the BMSC sheet than in cultured BMSCs (P <0.05). Moreover, alizarin red staining quantification, ALP quantification and calcein blue fluorescence showed the osteogenic potential of BMSCs treated with PRP and calcium phosphate particles The implanted BMSCs were detectable at 1 day, 3 days, 7 days, 2 weeks and 4 weeks after surgery by a small animal in vivo fluorescence imaging system and were visualized in the defect zones by confocal microscopy. At 4 weeks after implantation, the defects treated with the BMSC sheet/PRP gel/calcium phosphate particles showed significantly more bone formation than the other five groups.
Conclusions: Incorporation of an BMSC sheet into the PRP gel/calcium phosphate particles greatly promoted bone regeneration. These BMSC sheet and tissue engineering strategies offer therapeutic opportunities for promoting bone defect repair clinically.
Figures
Similar articles
-
Bone marrow mesenchymal stem cells, platelet-rich plasma and nanohydroxyapatite-type I collagen beads were integral parts of biomimetic bone substitutes for bone regeneration.J Tissue Eng Regen Med. 2013 Nov;7(11):841-54. doi: 10.1002/term.1472. Epub 2012 Jun 28. J Tissue Eng Regen Med. 2013. PMID: 22744907
-
Bone regeneration in minipigs via calcium phosphate cement scaffold delivering autologous bone marrow mesenchymal stem cells and platelet-rich plasma.J Tissue Eng Regen Med. 2018 Feb;12(2):e937-e948. doi: 10.1002/term.2416. Epub 2017 Jun 2. J Tissue Eng Regen Med. 2018. PMID: 28102000
-
Incorporating platelet-rich plasma into coaxial electrospun nanofibers for bone tissue engineering.Int J Pharm. 2018 Aug 25;547(1-2):656-666. doi: 10.1016/j.ijpharm.2018.06.020. Epub 2018 Jun 7. Int J Pharm. 2018. PMID: 29886100
-
Biomedical application of low molecular weight heparin/protamine nano/micro particles as cell- and growth factor-carriers and coating matrix.Int J Mol Sci. 2015 May 22;16(5):11785-803. doi: 10.3390/ijms160511785. Int J Mol Sci. 2015. PMID: 26006248 Free PMC article. Review.
-
Periosteum and development of the tissue-engineered periosteum for guided bone regeneration.J Orthop Translat. 2022 Feb 16;33:41-54. doi: 10.1016/j.jot.2022.01.002. eCollection 2022 Mar. J Orthop Translat. 2022. PMID: 35228996 Free PMC article. Review.
Cited by
-
The effect of LyPRP/collagen composite hydrogel on osteogenic differentiation of rBMSCs.Regen Biomater. 2020 Dec 11;8(1):rbaa053. doi: 10.1093/rb/rbaa053. eCollection 2021 Feb 1. Regen Biomater. 2020. PMID: 33732498 Free PMC article.
-
A Comprehensive Review of Platelet-Rich Plasma and Its Emerging Role in Accelerating Bone Healing.Cureus. 2024 Feb 13;16(2):e54122. doi: 10.7759/cureus.54122. eCollection 2024 Feb. Cureus. 2024. PMID: 38487114 Free PMC article. Review.
-
Platelet-rich plasma for the treatment of bone defects: from pre-clinical rational to evidence in the clinical practice. A systematic review.Int Orthop. 2017 Feb;41(2):221-237. doi: 10.1007/s00264-016-3342-9. Epub 2016 Nov 26. Int Orthop. 2017. PMID: 27888295 Review.
-
Autogenous bone particles combined with platelet-rich plasma can stimulate bone regeneration in rabbits.Exp Ther Med. 2020 Dec;20(6):279. doi: 10.3892/etm.2020.9409. Epub 2020 Oct 27. Exp Ther Med. 2020. PMID: 33200004 Free PMC article.
-
The platelet-rich plasma and mesenchymal stem cell milieu: A review of therapeutic effects on bone healing.J Orthop Res. 2020 Dec;38(12):2539-2550. doi: 10.1002/jor.24786. Epub 2020 Jul 17. J Orthop Res. 2020. PMID: 32589800 Free PMC article. Review.
References
-
- Kon E, Muraglia A, Corsi A, Bianco P, Marcacci M, Martin I, et al. Autologous bone marrow stromal cells loaded onto porous hydroxyapatite ceramic accelerate bone repair in critical-size defects of sheep long bones. J Biomed Mater Res. 2000;49:328–37. doi: 10.1002/(SICI)1097-4636(20000305)49:3<328::AID-JBM5>3.0.CO;2-Q. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

