Peripheral T Follicular Helper Cells Are the Major HIV Reservoir within Central Memory CD4 T Cells in Peripheral Blood from Chronically HIV-Infected Individuals on Combination Antiretroviral Therapy
- PMID: 26676775
- PMCID: PMC4810658
- DOI: 10.1128/JVI.02883-15
Peripheral T Follicular Helper Cells Are the Major HIV Reservoir within Central Memory CD4 T Cells in Peripheral Blood from Chronically HIV-Infected Individuals on Combination Antiretroviral Therapy
Abstract
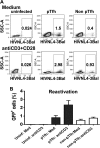
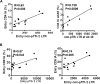
In this study, we examined the peripheral blood (PB) central memory (TCM) CD4(+) T cell subsets designated peripheral T follicular helper cells (pTfh cells) and non-pTfh cells to assess HIV permissiveness and persistence. Purified pTfh and non-pTfh cells from healthy HIV-negative donors were tested for HIV permissiveness using green fluorescent protein (GFP)-expressing HIV-1NL4-3/Ba-L, followed by viral reactivation using beads coated with anti-CD3/anti-CD28 monoclonal antibodies. The role of pTfh cells in HIV persistence was analyzed in 12 chronically HIV-1 infected patients before and 48 weeks after initiation of raltegravir-containing combination antiretroviral therapy (cART). Total cellular HIV-1 DNA and episomes containing two copies of the viral long terminal repeat (2LTR circles) were analyzed in using droplet digital PCR in the purified pTfh and non-pTfh cells. Activation-inducible HIV p24 expression was determined by flow cytometry. Results indicate that pTfh cells, in particular PD1(+) pTfh cells, showed greater permissiveness for HIV infection than non-pTfh cells. At week 48 on cART, HIV DNA levels were unchanged from pre-cART levels, although a significant decrease in 2LTR circles was observed in both cell subsets. Inducible HIV p24 expression was higher in pTfh cells than in non-pTfh cells, with the highest frequencies in the PD1(+) CXCR3(-) pTfh cell subset. Frequencies of HLADR(+) CD38(+) activated CD4 T cells correlated with 2LTR circles in pTfh and non-pTfh cells at both time points and with p24(+) cells at entry. In conclusion, among CD4 TCM cells in PB of aviremic patients on cART, pTfh cells, in particular the PD1(+) CXCR3(-) subset, constitute a major HIV reservoir that is sustained by ongoing residual immune activation. The inducible HIV p24 assay is useful for monitoring HIV reservoirs in defined CD4 T cell subsets.
Importance: Identification of the type and nature of the cellular compartments of circulating HIV reservoirs is important for targeting of HIV cure strategies. In lymph nodes (LN), a subset of CD4 T cells called T follicular helper (Tfh) cells are preferentially infected by HIV. Central memory (TCM) CD4 T cells are the major cellular reservoir for HIV in peripheral blood and contain a subset of CD4 TCM cells expressing chemokine receptor CXCR5 similar in function to LN Tfh cells termed peripheral Tfh (pTfh) cells. We found that the circulating pTfh cells are highly susceptible to HIV infection and that in HIV-infected patients, HIV persists in these cells following plasma virus suppression with potent cART. These pTfh cells, which constitute a subset of TCM CD4 T cells, can be readily monitored in peripheral blood to assess HIV persistence.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures






Similar articles
-
Peripheral T follicular helper Cells Make a Difference in HIV Reservoir Size between Elite Controllers and Patients on Successful cART.Sci Rep. 2017 Dec 1;7(1):16799. doi: 10.1038/s41598-017-17057-y. Sci Rep. 2017. PMID: 29196729 Free PMC article.
-
Low Peripheral T Follicular Helper Cells in Perinatally HIV-Infected Children Correlate With Advancing HIV Disease.Front Immunol. 2018 Aug 24;9:1901. doi: 10.3389/fimmu.2018.01901. eCollection 2018. Front Immunol. 2018. PMID: 30197641 Free PMC article.
-
Genetic Diversity, Compartmentalization, and Age of HIV Proviruses Persisting in CD4+ T Cell Subsets during Long-Term Combination Antiretroviral Therapy.J Virol. 2020 Feb 14;94(5):e01786-19. doi: 10.1128/JVI.01786-19. Print 2020 Feb 14. J Virol. 2020. PMID: 31776273 Free PMC article.
-
The role of integration and clonal expansion in HIV infection: live long and prosper.Retrovirology. 2018 Oct 23;15(1):71. doi: 10.1186/s12977-018-0448-8. Retrovirology. 2018. PMID: 30352600 Free PMC article. Review.
-
Comprehensive Mass Cytometry Analysis of Cell Cycle, Activation, and Coinhibitory Receptors Expression in CD4 T Cells from Healthy and HIV-Infected Individuals.Cytometry B Clin Cytom. 2017 Jan;92(1):21-32. doi: 10.1002/cyto.b.21502. Cytometry B Clin Cytom. 2017. PMID: 27997758 Review.
Cited by
-
HIV-Infected Hepatic Stellate Cells or HCV-Infected Hepatocytes Are Unable to Promote Latency Reversal among HIV-Infected Mononuclear Cells.Pathogens. 2024 Feb 1;13(2):134. doi: 10.3390/pathogens13020134. Pathogens. 2024. PMID: 38392872 Free PMC article.
-
Tissue reservoirs of HIV.Curr Opin HIV AIDS. 2016 Jul;11(4):362-70. doi: 10.1097/COH.0000000000000293. Curr Opin HIV AIDS. 2016. PMID: 27259045 Free PMC article. Review.
-
Human Immunodeficiency Virus 1 (HIV-1): Viral Latency, the Reservoir, and the Cure.Yale J Biol Med. 2020 Sep 30;93(4):549-560. eCollection 2020 Sep. Yale J Biol Med. 2020. PMID: 33005119 Free PMC article. Review.
-
CD32 Expression is not Associated to HIV-DNA content in CD4 cell subsets of individuals with Different Levels of HIV Control.Sci Rep. 2018 Oct 19;8(1):15541. doi: 10.1038/s41598-018-33749-5. Sci Rep. 2018. PMID: 30341387 Free PMC article.
-
X4-Tropic Latent HIV-1 Is Enriched in Peripheral Follicular Helper T Cells and Is Correlated with Disease Progression.J Virol. 2020 Jan 6;94(2):e01219-19. doi: 10.1128/JVI.01219-19. Print 2020 Jan 6. J Virol. 2020. PMID: 31666372 Free PMC article. Clinical Trial.
References
-
- Palmer S, Maldarelli F, Wiegand A, Bernstein B, Hanna GJ, Brun SC, Kempf DJ, Mellors JW, Coffin JM, King MS. 2008. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc Natl Acad Sci U S A 105:3879–3884. doi:10.1073/pnas.0800050105. - DOI - PMC - PubMed
-
- Dinoso JB, Kim SY, Wiegand AM, Palmer SE, Gange SJ, Cranmer L, O'Shea A, Callender M, Spivak A, Brennan T, Kearney MF, Proschan MA, Mican JM, Rehm CA, Coffin JM, Mellors JW, Siliciano RF, Maldarelli F. 2009. Treatment intensification does not reduce residual HIV-1 viremia in patients on highly active antiretroviral therapy. Proc Natl Acad Sci U S A 106:9403–9408. doi:10.1073/pnas.0903107106. - DOI - PMC - PubMed
-
- Gandhi RT, Coombs RW, Chan ES, Bosch RJ, Zheng L, Margolis DM, Read S, Kallungal B, Chang M, Goecker EA, Wiegand A, Kearney M, Jacobson JM, D'Aquila R, Lederman MM, Mellors JW, Eron JJ. 2012. No effect of raltegravir intensification on viral replication markers in the blood of HIV-1-infected patients receiving antiretroviral therapy. J Acquir Immune Defic Syndr 59:229–235. doi:10.1097/QAI.0b013e31823fd1f2. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

