Clinical features of TBK1 carriers compared with C9orf72, GRN and non-mutation carriers in a Belgian cohort
- PMID: 26674655
- PMCID: PMC4805085
- DOI: 10.1093/brain/awv358
Clinical features of TBK1 carriers compared with C9orf72, GRN and non-mutation carriers in a Belgian cohort
Abstract
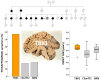
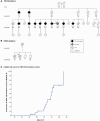
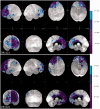
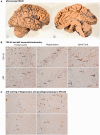
We identified in a cohort of patients with frontotemporal dementia (n = 481) or amyotrophic lateral sclerosis (n = 147), 10 index patients carrying a TBK1 loss of function mutation reducing TBK1 expression by 50%. Here, we describe the clinical and pathological characteristics of the 10 index patients and six of their affected relatives carrying a TBK1 mutation. Six TBK1 carriers were diagnosed with frontotemporal dementia, seven with amyotrophic lateral sclerosis, one with both clinical phenotypes and two with dementia unspecified. The mean age at onset of all 16 TBK1 carriers was 62.1 ± 8.9 years (range 41-73) with a mean disease duration of 4.7 ± 4.5 years (range 1-13). TBK1 carriers with amyotrophic lateral sclerosis had shorter disease duration than carriers with frontotemporal dementia. Six of seven TBK1 carriers were diagnosed with the behavioural variant of frontotemporal dementia, presenting predominantly as disinhibition. Memory loss was an important associated symptom in the initial phase of the disease in all but one of the carriers with frontotemporal dementia. Three of the patients with amyotrophic lateral sclerosis exhibited pronounced upper motor neuron symptoms. Overall, neuroimaging displayed widespread atrophy, both symmetric and asymmetric. Brain perfusion single-photon emission computed tomography or fluorodeoxyglucose-positron emission tomography showed asymmetric and predominantly frontotemporal involvement. Neuropathology in two patients demonstrated TDP-43 type B pathology. Further, we compared genotype-phenotype data of TBK1 carriers with frontotemporal dementia (n = 7), with those of frontotemporal dementia patients with a C9orf72 repeat expansion (n = 65) or a GRN mutation (n = 52) and with frontotemporal dementia patients (n = 259) negative for mutations in currently known causal genes. TBK1 carriers with frontotemporal dementia had a later age at onset (63.3 years) than C9orf72 carriers (54.3 years) (P = 0.019). In clear contrast with TBK1 carriers, GRN carriers were more often diagnosed with the language variant than the behavioural variant, and presented in case of the diagnosis of behavioural variant, more often than TBK1 carriers with apathy as the predominant characteristic (P = 0.004). Also, TBK1 carriers exhibited more often extrapyramidal symptoms than C9orf72 carriers (P = 0.038). In conclusion, our study identified clinical differences between the TBK1, C9orf72 and GRN carriers, which allows us to formulate guidelines for genetic diagnosis. After a negative result for C9orf72, patients with both frontotemporal dementia and amyotrophic lateral sclerosis should be tested first for mutations in TBK1. Specifically in frontotemporal dementia patients with early memory difficulties, a relatively late age at onset or extrapyramidal symptoms, screening for TBK1 mutations should be considered.
Keywords: C9orf72; TBK1; amyotrophic lateral sclerosis; frontotemporal lobar degeneration; genotype–phenotype correlations.
© The Author (2015). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures
Similar articles
-
The clinical and pathological phenotype of C9ORF72 hexanucleotide repeat expansions.Brain. 2012 Mar;135(Pt 3):723-35. doi: 10.1093/brain/awr353. Epub 2012 Feb 1. Brain. 2012. PMID: 22300876
-
Distinct clinical characteristics of C9orf72 expansion carriers compared with GRN, MAPT, and nonmutation carriers in a Flanders-Belgian FTLD cohort.JAMA Neurol. 2013 Mar 1;70(3):365-73. doi: 10.1001/2013.jamaneurol.181. JAMA Neurol. 2013. PMID: 23338682
-
Co-occurrence of the C9ORF72 expansion and a novel GRN mutation in a family with alternative expression of frontotemporal dementia and amyotrophic lateral sclerosis.J Alzheimers Dis. 2015;44(1):49-56. doi: 10.3233/JAD-141794. J Alzheimers Dis. 2015. PMID: 25182743
-
Sex differences in the prevalence of genetic mutations in FTD and ALS: A meta-analysis.Neurology. 2017 Oct 10;89(15):1633-1642. doi: 10.1212/WNL.0000000000004494. Epub 2017 Sep 15. Neurology. 2017. PMID: 28916533 Free PMC article. Review.
-
[Genetic coherence between hereditary amyotrophic lateral sclerosis and frontotemporal dementia].Tidsskr Nor Laegeforen. 2014 Feb 11;134(3):302-6. doi: 10.4045/tidsskr.13.0049. Tidsskr Nor Laegeforen. 2014. PMID: 24518478 Review. Norwegian.
Cited by
-
Novel Intronic Mutations of TBK1 Promote Aberrant Splicing Modes in Amyotrophic Lateral Sclerosis.Front Mol Neurosci. 2022 Feb 24;15:691534. doi: 10.3389/fnmol.2022.691534. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35283724 Free PMC article.
-
TDP43 ribonucleoprotein granules: physiologic function to pathologic aggregates.RNA Biol. 2021 Oct 15;18(sup1):128-138. doi: 10.1080/15476286.2021.1963099. Epub 2021 Aug 19. RNA Biol. 2021. PMID: 34412568 Free PMC article. Review.
-
Novel genes associated with amyotrophic lateral sclerosis: diagnostic and clinical implications.Lancet Neurol. 2018 Jan;17(1):94-102. doi: 10.1016/S1474-4422(17)30401-5. Epub 2017 Nov 16. Lancet Neurol. 2018. PMID: 29154141 Free PMC article. Review.
-
Role of the STING→IRF3 Pathway in Ambient GABA Homeostasis and Cognitive Function.J Neurosci. 2024 Oct 9;44(41):e1810232024. doi: 10.1523/JNEUROSCI.1810-23.2024. J Neurosci. 2024. PMID: 39227159
-
Diagnostic value of cerebrospinal fluid tau, neurofilament, and progranulin in definite frontotemporal lobar degeneration.Alzheimers Res Ther. 2018 Mar 20;10(1):31. doi: 10.1186/s13195-018-0364-0. Alzheimers Res Ther. 2018. PMID: 29559004 Free PMC article.
References
-
- Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 2006; 442: 916–19. - PubMed
-
- Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. [Review]. Amyotroph Lateral Scler Other Motor Neuron Disord 2000; 1: 293–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

