Enrichment and characterization of ferritin for nanomaterial applications
- PMID: 26656976
- PMCID: PMC6284806
- DOI: 10.1088/0957-4484/27/4/045102
Enrichment and characterization of ferritin for nanomaterial applications
Abstract
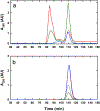
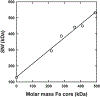
Ferritin is a ubiquitous iron storage protein utilized as a nanomaterial for labeling biomolecules and nanoparticle construction. Commercially available preparations of horse spleen ferritin, widely used as a starting material, contain a distribution of ferritins with different iron loads. We describe a detailed approach to the enrichment of differentially loaded ferritin molecules by common biophysical techniques such as size exclusion chromatography and preparative ultracentrifugation, and characterize these preparations by dynamic light scattering, and analytical ultracentrifugation. We demonstrate a combination of methods to standardize an approach for determining the chemical load of nearly any particle, including nanoparticles and metal colloids. Purification and characterization of iron content in monodisperse ferritin species is particularly critical for several applications in nanomaterial science.
Figures
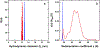
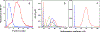
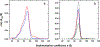

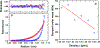
Similar articles
-
Purification and characterization of liver ferritins from different animal species.Vet Res Commun. 1999 May;23(3):165-81. doi: 10.1023/a:1006225601199. Vet Res Commun. 1999. PMID: 10401720
-
Fractionation of horse spleen ferritin. Relationship of Fe-N-ratio to some physico-chemical properties.Hoppe Seylers Z Physiol Chem. 1969 Nov;350(11):1331-9. doi: 10.1515/bchm2.1969.350.2.1331. Hoppe Seylers Z Physiol Chem. 1969. PMID: 5391427 No abstract available.
-
Size control for two-dimensional iron oxide nanodots derived from biological molecules.J Colloid Interface Sci. 2006 Jul 15;299(2):761-5. doi: 10.1016/j.jcis.2006.02.022. Epub 2006 Mar 22. J Colloid Interface Sci. 2006. PMID: 16554058
-
The sedimentation properties of ferritins. New insights and analysis of methods of nanoparticle preparation.Biochim Biophys Acta. 2010 Aug;1800(8):858-70. doi: 10.1016/j.bbagen.2010.03.012. Epub 2010 Mar 20. Biochim Biophys Acta. 2010. PMID: 20307627 Free PMC article. Review.
-
Ferritin in the field of nanodevices.Biochim Biophys Acta. 2010 Aug;1800(8):846-57. doi: 10.1016/j.bbagen.2010.03.005. Epub 2010 Mar 12. Biochim Biophys Acta. 2010. PMID: 20227466 Review.
Cited by
-
FTH1 Pseudogenes in Cancer and Cell Metabolism.Cells. 2020 Nov 28;9(12):2554. doi: 10.3390/cells9122554. Cells. 2020. PMID: 33260500 Free PMC article. Review.
-
Functional ferritin nanoparticles for biomedical applications.Front Chem Sci Eng. 2017 Dec;11(4):633-646. doi: 10.1007/s11705-017-1620-8. Epub 2017 Feb 15. Front Chem Sci Eng. 2017. PMID: 29503759 Free PMC article.
-
Multiple analyses of protein dynamics in solution.Biophys Rev. 2018 Apr;10(2):299-306. doi: 10.1007/s12551-017-0354-7. Epub 2017 Dec 4. Biophys Rev. 2018. PMID: 29204883 Free PMC article. Review.
References
-
- Chasteen ND, and Harrison PM (1999) Mineralization in ferritin: An efficient means of iron storage. J. Struct. Biol 126, 182–194. - PubMed
-
- Halliday JW, and Powell LW (1984) Ferritin metabolism and the liver. Seminars Liver Disease 4, 207–216. - PubMed
-
- Ford GC, Harrison PM, Rice DW, Smith JMA, Treffrey A, White JL, and Yariv J (1984) Ferritin: Design and Formation of an iron-storage molecule. Phil. Trans. R. Soc. Lond 304, 551–565. - PubMed
-
- Harrison PM, and Arosio P (1996) The ferritins: molecular properties, iron storage function and cellular regulation. Biochim. Biophys. Acta 1275, 161–203. - PubMed
-
- Crichton RR, and Charloteaux-Wauters M (1987) Iron transport and storage. Eur. J. Biochem 164, 485–506. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
