Acquisition and Longevity of Antibodies to Plasmodium vivax Preerythrocytic Antigens in Western Thailand
- PMID: 26656115
- PMCID: PMC4744911
- DOI: 10.1128/CVI.00501-15
Acquisition and Longevity of Antibodies to Plasmodium vivax Preerythrocytic Antigens in Western Thailand
Abstract
Plasmodium vivax is now the dominant Plasmodium species causing malaria in Thailand, yet little is known about naturally acquired immune responses to this parasite in this low-transmission region. The preerythrocytic stage of the P. vivax life cycle is considered an excellent target for a malaria vaccine, and in this study, we assessed the stability of the seropositivity and the magnitude of IgG responses to three different preerythrocytic P. vivax proteins in two groups of adults from a region of western Thailand where malaria is endemic. These individuals were enrolled in a yearlong cohort study, which comprised one group that remained P. vivax free (by quantitative PCR [qPCR] detection, n = 31) and another that experienced two or more blood-stage P. vivax infections during the year of follow up (n = 31). Despite overall low levels of seropositivity, IgG positivity and magnitude were long-lived over the 1-year period in the absence of qPCR-detectable blood-stage P. vivax infections. In contrast, in the adults with two or more P. vivax infections during the year, IgG positivity was maintained, but the magnitude of the response to P. vivax circumsporozoite protein 210 (CSP210) decreased over time. These findings demonstrate that long-term humoral immunity can develop in low-transmission regions.
Copyright © 2016 Longley et al.
Figures


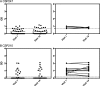
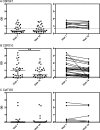
Similar articles
-
Evaluation of naturally acquired immune responses against novel pre-erythrocytic Plasmodium vivax proteins in a low endemic malaria population located in the Peruvian Amazon Basin.Malar J. 2024 May 23;23(1):163. doi: 10.1186/s12936-024-04978-z. Malar J. 2024. PMID: 38783317 Free PMC article.
-
Evaluation of Naturally Acquired Antibody Responses to Two Variant Forms of Plasmodium vivax Apical Membrane Antigen-1 in Individuals Living in Areas of Low and Unstable Malaria Transmission of Iran.Arch Iran Med. 2015 Dec;18(12):834-43. Arch Iran Med. 2015. PMID: 26621016
-
Malaria transmission and individual variability of the naturally acquired IgG antibody against the Plasmodium vivax blood-stage antigen in an endemic area in Brazil.Acta Trop. 2020 Sep;209:105537. doi: 10.1016/j.actatropica.2020.105537. Epub 2020 May 23. Acta Trop. 2020. PMID: 32454033
-
Development and longevity of naturally acquired antibody and memory B cell responses against Plasmodium vivax infection.PLoS Negl Trop Dis. 2024 Oct 24;18(10):e0012600. doi: 10.1371/journal.pntd.0012600. eCollection 2024 Oct. PLoS Negl Trop Dis. 2024. PMID: 39446698 Free PMC article. Review.
-
The immunology of Plasmodium vivax malaria.Immunol Rev. 2020 Jan;293(1):163-189. doi: 10.1111/imr.12816. Epub 2019 Oct 23. Immunol Rev. 2020. PMID: 31642531 Review.
Cited by
-
Plasmodium vivax serological exposure markers: PvMSP1-42-induced humoral and memory B-cell response generates long-lived antibodies.PLoS Pathog. 2024 Jun 28;20(6):e1012334. doi: 10.1371/journal.ppat.1012334. eCollection 2024 Jun. PLoS Pathog. 2024. PMID: 38941356 Free PMC article.
-
Identification of novel Plasmodium vivax proteins associated with protection against clinical malaria.Front Cell Infect Microbiol. 2023 Jan 25;13:1076150. doi: 10.3389/fcimb.2023.1076150. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 36761894 Free PMC article.
-
Evaluation of naturally acquired immune responses against novel pre-erythrocytic Plasmodium vivax proteins in a low endemic malaria population located in the Peruvian Amazon Basin.Malar J. 2024 May 23;23(1):163. doi: 10.1186/s12936-024-04978-z. Malar J. 2024. PMID: 38783317 Free PMC article.
-
Asymptomatic Plasmodium vivax infections induce robust IgG responses to multiple blood-stage proteins in a low-transmission region of western Thailand.Malar J. 2017 Apr 28;16(1):178. doi: 10.1186/s12936-017-1826-8. Malar J. 2017. PMID: 28454546 Free PMC article.
-
Longitudinal analysis of antibody responses to Plasmodium vivax sporozoite antigens following natural infection.PLoS Negl Trop Dis. 2024 Jan 26;18(1):e0011907. doi: 10.1371/journal.pntd.0011907. eCollection 2024 Jan. PLoS Negl Trop Dis. 2024. PMID: 38277340 Free PMC article.
References
-
- Gething PW, Elyazar IR, Moyes CL, Smith DL, Battle KE, Guerra CA, Patil AP, Tatem AJ, Howes RE, Myers MF, George DB, Horby P, Wertheim HF, Price RN, Mueller I, Baird JK, Hay SI. 2012. A long neglected world malaria map: Plasmodium vivax endemicity in 2010. PLoS Negl Trop Dis 6:e1814. doi:10.1371/journal.pntd.0001814. - DOI - PMC - PubMed
-
- Phimpraphi W, Paul RE, Yimsamran S, Puangsa-art S, Thanyavanich N, Maneeboonyang W, Prommongkol S, Sornklom S, Chaimungkun W, Chavez IF, Blanc H, Looareesuwan S, Sakuntabhai A, Singhasivanon P. 2008. Longitudinal study of Plasmodium falciparum and Plasmodium vivax in a Karen population in Thailand. Malar J 7:99. doi:10.1186/1475-2875-7-99. - DOI - PMC - PubMed
-
- Gunewardena DM, Carter R, Mendis KN. 1994. Patterns of acquired anti-malarial immunity in Sri Lanka. Mem Inst Oswaldo Cruz 89(Suppl):S63–S65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

