Hyperpolarization-independent maturation and refinement of GABA/glycinergic connections in the auditory brain stem
- PMID: 26655825
- PMCID: PMC4808136
- DOI: 10.1152/jn.00926.2015
Hyperpolarization-independent maturation and refinement of GABA/glycinergic connections in the auditory brain stem
Abstract
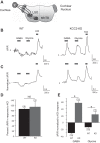
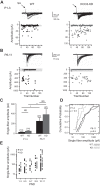
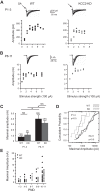
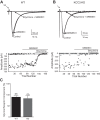
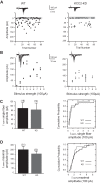
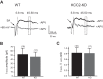
During development GABA and glycine synapses are initially excitatory before they gradually become inhibitory. This transition is due to a developmental increase in the activity of neuronal potassium-chloride cotransporter 2 (KCC2), which shifts the chloride equilibrium potential (ECl) to values more negative than the resting membrane potential. While the role of early GABA and glycine depolarizations in neuronal development has become increasingly clear, the role of the transition to hyperpolarization in synapse maturation and circuit refinement has remained an open question. Here we investigated this question by examining the maturation and developmental refinement of GABA/glycinergic and glutamatergic synapses in the lateral superior olive (LSO), a binaural auditory brain stem nucleus, in KCC2-knockdown mice, in which GABA and glycine remain depolarizing. We found that many key events in the development of synaptic inputs to the LSO, such as changes in neurotransmitter phenotype, strengthening and elimination of GABA/glycinergic connection, and maturation of glutamatergic synapses, occur undisturbed in KCC2-knockdown mice compared with wild-type mice. These results indicate that maturation of inhibitory and excitatory synapses in the LSO is independent of the GABA and glycine depolarization-to-hyperpolarization transition.
Keywords: auditory brain stem development; inhibitory plasticity; potassium-chloride cotransporter.
Copyright © 2016 the American Physiological Society.
Figures

Similar articles
-
VGLUT3 does not synergize GABA/glycine release during functional refinement of an inhibitory auditory circuit.Front Neural Circuits. 2014 Nov 26;8:140. doi: 10.3389/fncir.2014.00140. eCollection 2014. Front Neural Circuits. 2014. PMID: 25505386 Free PMC article.
-
Glycinergic and GABAergic calcium responses in the developing lateral superior olive.Eur J Neurosci. 2002 Apr;15(7):1093-104. doi: 10.1046/j.1460-9568.2002.01946.x. Eur J Neurosci. 2002. PMID: 11982621 Free PMC article.
-
KCC2 knockdown impairs glycinergic synapse maturation in cultured spinal cord neurons.Histochem Cell Biol. 2016 Jun;145(6):637-46. doi: 10.1007/s00418-015-1397-0. Epub 2016 Jan 16. Histochem Cell Biol. 2016. PMID: 26780567
-
Developmental Formation of the GABAergic and Glycinergic Networks in the Mouse Spinal Cord.Int J Mol Sci. 2022 Jan 13;23(2):834. doi: 10.3390/ijms23020834. Int J Mol Sci. 2022. PMID: 35055019 Free PMC article. Review.
-
K-Cl cotransporter KCC2--a moonlighting protein in excitatory and inhibitory synapse development and function.Pflugers Arch. 2015 Apr;467(4):615-24. doi: 10.1007/s00424-014-1547-6. Epub 2014 Jun 10. Pflugers Arch. 2015. PMID: 24909111 Review.
Cited by
-
Synapse Maturation and Developmental Impairment in the Medial Nucleus of the Trapezoid Body.Front Integr Neurosci. 2022 Feb 9;16:804221. doi: 10.3389/fnint.2022.804221. eCollection 2022. Front Integr Neurosci. 2022. PMID: 35221938 Free PMC article. Review.
-
Assembly and maintenance of GABAergic and Glycinergic circuits in the mammalian nervous system.Neural Dev. 2018 Jun 7;13(1):12. doi: 10.1186/s13064-018-0109-6. Neural Dev. 2018. PMID: 29875009 Free PMC article. Review.
-
Octopus Cells in the Posteroventral Cochlear Nucleus Provide the Main Excitatory Input to the Superior Paraolivary Nucleus.Front Neural Circuits. 2017 May 31;11:37. doi: 10.3389/fncir.2017.00037. eCollection 2017. Front Neural Circuits. 2017. PMID: 28620283 Free PMC article.
-
Chronic intermittent ethanol promotes ventral subiculum hyperexcitability via increases in extrinsic basolateral amygdala input and local network activity.Sci Rep. 2021 Apr 22;11(1):8749. doi: 10.1038/s41598-021-87899-0. Sci Rep. 2021. PMID: 33888757 Free PMC article.
-
Nitric Oxide Signaling in the Auditory Pathway.Front Neural Circuits. 2021 Oct 12;15:759342. doi: 10.3389/fncir.2021.759342. eCollection 2021. Front Neural Circuits. 2021. PMID: 34712124 Free PMC article. Review.
References
-
- Awatramani GB, Turecek R, Trussell LO. Staggered development of GABAergic and glycinergic transmission in the MNTB. J Neurophysiol 93: 819–828, 2005. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

