Nanotechnology-Based Approaches for Guiding Neural Regeneration
- PMID: 26653885
- PMCID: PMC5808885
- DOI: 10.1021/acs.accounts.5b00345
Nanotechnology-Based Approaches for Guiding Neural Regeneration
Abstract
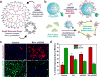
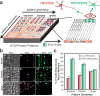
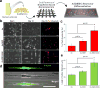
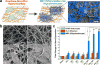
The mammalian brain is a phenomenal piece of "organic machinery" that has fascinated scientists and clinicians for centuries. The intricate network of tens of billions of neurons dispersed in a mixture of chemical and biochemical constituents gives rise to thoughts, feelings, memories, and life as we know it. In turn, subtle imbalances or damage to this system can cause severe complications in physical, motor, psychological, and cognitive function. Moreover, the inevitable loss of nerve tissue caused by degenerative diseases and traumatic injuries is particularly devastating because of the limited regenerative capabilities of the central nervous system (i.e., the brain and spinal cord). Among current approaches, stem-cell-based regenerative medicine has shown the greatest promise toward repairing and regenerating destroyed neural tissue. However, establishing controlled and reliable methodologies to guide stem cell differentiation into specialized neural cells of interest (e.g., neurons and oligodendrocytes) has been a prevailing challenge in the field. In this Account, we summarize the nanotechnology-based approaches our group has recently developed to guide stem-cell-based neural regeneration. We focus on three overarching strategies that were adopted to selectively control this process. First, soluble microenvironmental factors play a critical role in directing the fate of stem cells. Multiple factors have been developed in the form of small-molecule drugs, biochemical analogues, and DNA/RNA-based vectors to direct neural differentiation. However, the delivery of these factors with high transfection efficiency and minimal cytotoxicity has been challenging, especially to sensitive cell lines such as stem cells. In our first approach, we designed nanoparticle-based systems for the efficient delivery of such soluble factors to control neural differentiation. Our nanoparticles, comprising either organic or inorganic elements, were biocompatible and offered multifunctional capabilities such as imaging and delivery. Moving from the soluble microenvironment in which cells are immersed to the underlying surface, cells can sense and consequently respond to the physical microenvironment in which they reside. For instance, changes in cell adhesion, shape, and spreading are key cellular responses to surface properties of the underlying substrate. In our second approach, we modulated the surface chemistry of two-dimensional substrates to control neural stem cell morphology and the resulting differentiation process. Patterned surfaces consisting of immobilized extracellular matrix (ECM) proteins and/or nanomaterials were generated and utilized to guide neuronal differentiation and polarization. In our third approach, building on the above-mentioned approaches, we further tuned the cell-ECM interactions by introducing nanotopographical features in the form of nanoparticle films or nanofiber scaffolds. Besides providing a three-dimensional surface topography, our unique nanoscaffolds were observed to enhance gene delivery, facilitate axonal alignment, and selectively control differentiation into neural cell lines of interest. Overall, nanotechnology-based approaches offer the precise physicochemical control required to generate tools suitable for applications in neuroscience.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Applications of Proteomics to Nerve Regeneration Research.In: Alzate O, editor. Neuroproteomics. Boca Raton (FL): CRC Press/Taylor & Francis; 2010. Chapter 15. In: Alzate O, editor. Neuroproteomics. Boca Raton (FL): CRC Press/Taylor & Francis; 2010. Chapter 15. PMID: 21882439 Free Books & Documents. Review.
-
Controlling the Outgrowth and Functions of Neural Stem Cells: The Effect of Surface Topography.Chemphyschem. 2018 May 22;19(10):1143-1163. doi: 10.1002/cphc.201701175. Epub 2018 Feb 19. Chemphyschem. 2018. PMID: 29457860 Review.
-
Nanofiber topography and sustained biochemical signaling enhance human mesenchymal stem cell neural commitment.Acta Biomater. 2012 Mar;8(3):1290-302. doi: 10.1016/j.actbio.2011.11.019. Epub 2011 Nov 20. Acta Biomater. 2012. PMID: 22154861
-
Nanotechnology Facilitated Cultured Neuronal Network and Its Applications.Int J Mol Sci. 2021 May 24;22(11):5552. doi: 10.3390/ijms22115552. Int J Mol Sci. 2021. PMID: 34074027 Free PMC article. Review.
-
Nanomaterials for Engineering Stem Cell Responses.Adv Healthc Mater. 2015 Aug 5;4(11):1600-27. doi: 10.1002/adhm.201500272. Epub 2015 May 26. Adv Healthc Mater. 2015. PMID: 26010739 Review.
Cited by
-
Nanotechnology for Neuroscience: Promising Approaches for Diagnostics, Therapeutics and Brain Activity Mapping.Adv Funct Mater. 2017 Oct 19;27(39):1700489. doi: 10.1002/adfm.201700489. Epub 2017 Aug 14. Adv Funct Mater. 2017. PMID: 30853878 Free PMC article.
-
Effects of nanofibers on mesenchymal stem cells: environmental factors affecting cell adhesion and osteogenic differentiation and their mechanisms.J Zhejiang Univ Sci B. 2020 Nov.;21(11):871-884. doi: 10.1631/jzus.B2000355. J Zhejiang Univ Sci B. 2020. PMID: 33150771 Free PMC article. Review.
-
A suspended carbon fiber culture to model myelination by human Schwann cells.J Mater Sci Mater Med. 2017 Apr;28(4):57. doi: 10.1007/s10856-017-5867-x. Epub 2017 Feb 16. J Mater Sci Mater Med. 2017. PMID: 28210970
-
Supramolecular Nanostructures Based on Cyclodextrin and Poly(ethylene oxide): Syntheses, Structural Characterizations and Applications for Drug Delivery.Polymers (Basel). 2016 May 17;8(5):198. doi: 10.3390/polym8050198. Polymers (Basel). 2016. PMID: 30979290 Free PMC article. Review.
-
Direct Conjugation of Retinoic Acid with Gold Nanoparticles to Improve Neural Differentiation of Human Adipose Stem Cells.J Mol Neurosci. 2020 Nov;70(11):1836-1850. doi: 10.1007/s12031-020-01577-w. Epub 2020 Jun 8. J Mol Neurosci. 2020. PMID: 32514739
References
-
- Lindvall O, Kokaia Z. Stem cells for the treatment of neurological disorders. Nature. 2006;441:1094–1096. - PubMed
-
- Aboody K, Capela A, Niazi N, Stern JH, Temple S. Translating Stem Cell Studies to the Clinic for CNS Repair: Current State of the Art and the Need for a Rosetta Stone. Neuron. 2011;70:597–613. - PubMed
-
- Martino G, Pluchino S. The therapeutic potential of neural stem cells. Nat Rev Neurosci. 2006;7:395–406. - PubMed
-
- Trueman RC, Klein A, Lindgren HS, Lelos MJ, Dunnett SB. Repair of the CNS using endogenous and transplanted neural stem cells. Curr. Top Behav Neurosci. 2012;15:357–398. - PubMed
-
- Kirkeby A, Grealish S, Wolf DA, Nelander J, Wood J, Lundblad M, Lindvall O, Parmar M. Generation of Regionally Specified Neural Progenitors and Functional Neurons from Human Embryonic Stem Cells under Defined Conditions. Cell Rep. 2012;1:703–714. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

