Advancing the use of Lactobacillus acidophilus surface layer protein A for the treatment of intestinal disorders in humans
- PMID: 26647142
- PMCID: PMC4826124
- DOI: 10.1080/19490976.2015.1107697
Advancing the use of Lactobacillus acidophilus surface layer protein A for the treatment of intestinal disorders in humans
Erratum in
- Addendum to: Lightfoot YL, Selle K, Yang T, Goh YJ, Sahay B, Zadeh M, Owen JL, Colliou N, Li E, Johannssen T, Lepenies B, Klaenhammer TR, Mohamadzadeh M. SIGNR3-dependent immune regulation by Lactobacillus acidophilus-Surface layer protein A in colitis. EMBO Journal 2015 Apr 1;34(7):881-95; PMID: 2566591; PMCID: PMC4388597.
Abstract
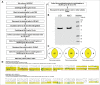
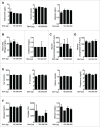
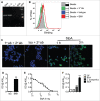
Intestinal immunity is subject to complex and fine-tuned regulation dictated by interactions of the resident microbial community and their gene products with host innate cells. Deterioration of this delicate process may result in devastating autoinflammatory diseases, including inflammatory bowel disease (IBD), which primarily comprises Crohn's disease (CD) and ulcerative colitis (UC). Efficacious interventions to regulate proinflammatory signals, which play critical roles in IBD, require further scientific investigation. We recently demonstrated that rebalancing intestinal immunity via the surface layer protein A (SlpA) from Lactobacillus acidophilus NCFM potentially represents a feasible therapeutic approach to restore intestinal homeostasis. To expand on these findings, we established a new method of purifying bacterial SlpA, a new SlpA-specific monoclonal antibody, and found no SlpA-associated toxicity in mice. Thus, these data may assist in our efforts to determine the immune regulatory efficacy of SlpA in humans.
Keywords: bacterial protein isolation; colonic inflammation; gut microbiota; intestinal immune regulation; surface layer protein A.
Figures
Similar articles
-
SIGNR3-dependent immune regulation by Lactobacillus acidophilus surface layer protein A in colitis.EMBO J. 2015 Apr 1;34(7):881-95. doi: 10.15252/embj.201490296. Epub 2015 Feb 9. EMBO J. 2015. PMID: 25666591 Free PMC article.
-
Lactobacillus acidophilus CP23 with weak immunomodulatory activity lacks anchoring structure for surface layer protein.J Biosci Bioeng. 2015 May;119(5):521-5. doi: 10.1016/j.jbiosc.2014.10.003. Epub 2014 Oct 28. J Biosci Bioeng. 2015. PMID: 25454604
-
In Vivo Transcriptome of Lactobacillus acidophilus and Colonization Impact on Murine Host Intestinal Gene Expression.mBio. 2021 Jan 26;12(1):e03399-20. doi: 10.1128/mBio.03399-20. mBio. 2021. PMID: 33500337 Free PMC article.
-
Intestinal microbiota and ulcerative colitis.J Infect Chemother. 2015 Nov;21(11):761-8. doi: 10.1016/j.jiac.2015.07.010. Epub 2015 Sep 4. J Infect Chemother. 2015. PMID: 26346678 Review.
-
Maintenance of gut homeostasis by the mucosal immune system.Proc Jpn Acad Ser B Phys Biol Sci. 2016;92(9):423-435. doi: 10.2183/pjab.92.423. Proc Jpn Acad Ser B Phys Biol Sci. 2016. PMID: 27840390 Free PMC article. Review.
Cited by
-
Irradiation-Induced Intestinal Damage Is Recovered by the Indigenous Gut Bacteria Lactobacillus acidophilus.Front Cell Infect Microbiol. 2020 Aug 18;10:415. doi: 10.3389/fcimb.2020.00415. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32974214 Free PMC article.
-
Development of a Limosilactobacillus reuteri therapeutic delivery platform with reduced colonization potential.Appl Environ Microbiol. 2024 Nov 20;90(11):e0031224. doi: 10.1128/aem.00312-24. Epub 2024 Oct 31. Appl Environ Microbiol. 2024. PMID: 39480094 Free PMC article.
-
Surface layer proteins in species of the family Lactobacillaceae.Microb Biotechnol. 2023 Jun;16(6):1232-1249. doi: 10.1111/1751-7915.14230. Epub 2023 Feb 8. Microb Biotechnol. 2023. PMID: 36752119 Free PMC article.
-
Lactobacillus acidophilus alleviates pouchitis after ileal pouch-anal anastomosis in rats.World J Gastroenterol. 2017 Jul 14;23(26):4735-4743. doi: 10.3748/wjg.v23.i26.4735. World J Gastroenterol. 2017. PMID: 28765694 Free PMC article.
-
The molecular architecture of Lactobacillus S-layer: Assembly and attachment to teichoic acids.Proc Natl Acad Sci U S A. 2024 Jun 11;121(24):e2401686121. doi: 10.1073/pnas.2401686121. Epub 2024 Jun 5. Proc Natl Acad Sci U S A. 2024. PMID: 38838019 Free PMC article.
References
-
- Kappelman MD, Rifas-Shiman SL, Kleinman K, Ollendorf D, Bousvaros A, Grand RJ, Finkelstein JA. The prevalence and geographic distribution of Crohn's disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol 2007; 5:1424-9; PMID:17904915; http://dx.doi.org/10.1016/j.cgh.2007.07.012 - DOI - PubMed
-
- Kappelman MD, Moore KR, Allen JK, Cook SF. Recent trends in the prevalence of Crohn's disease and ulcerative colitis in a commercially insured US population. Dig Dis Sci 2013; 58:519-25; PMID:22926499; http://dx.doi.org/10.1007/s10620-012-2371-5 - DOI - PMC - PubMed
-
- Walsh CJ, Guinane CM, O'Toole PW, Cotter PD. Beneficial modulation of the gut microbiota. FEBS Lett 2014; 588:4120-30; PMID:24681100; http://dx.doi.org/10.1016/j.febslet.2014.03.035 - DOI - PubMed
-
- Major G, Spiller R. Irritable bowel syndrome, inflammatory bowel disease and the microbiome. Curr Opin Endocrinol Diabetes Obes 2014; 21:15-21; PMID:24296462; http://dx.doi.org/10.1097/MED.0000000000000032 - DOI - PMC - PubMed
-
- Lee YK, Mazmanian SK. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science 2010; 330:1768-73; PMID:21205662; http://dx.doi.org/10.1126/science.1195568 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
