Stem Cell-Based Cell Carrier for Targeted Oncolytic Virotherapy: Translational Opportunity and Open Questions
- PMID: 26633462
- PMCID: PMC4690850
- DOI: 10.3390/v7122921
Stem Cell-Based Cell Carrier for Targeted Oncolytic Virotherapy: Translational Opportunity and Open Questions
Abstract
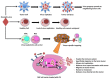
Oncolytic virotherapy for cancer is an innovative therapeutic option where the ability of a virus to promote cell lysis is harnessed and reprogrammed to selectively destroy cancer cells. Such treatment modalities exhibited antitumor activity in preclinical and clinical settings and appear to be well tolerated when tested in clinical trials. However, the clinical success of oncolytic virotherapy has been significantly hampered due to the inability to target systematic metastasis. This is partly due to the inability of the therapeutic virus to survive in the patient circulation, in order to target tumors at distant sites. An early study from various laboratories demonstrated that cells infected with oncolytic virus can protect the therapeutic payload form the host immune system as well as function as factories for virus production and enhance the therapeutic efficacy of oncolytic virus. While a variety of cell lineages possessed potential as cell carriers, copious investigation has established stem cells as a very attractive cell carrier system in oncolytic virotherapy. The ideal cell carrier desire to be susceptible to viral infection as well as support viral infection, maintain immunosuppressive properties to shield the loaded viruses from the host immune system, and most importantly possess an intrinsic tumor homing ability to deliver loaded viruses directly to the site of the metastasis-all qualities stem cells exhibit. In this review, we summarize the recent work in the development of stem cell-based carrier for oncolytic virotherapy, discuss the advantages and disadvantages of a variety of cell carriers, especially focusing on why stem cells have emerged as the leading candidate, and finally propose a future direction for stem cell-based targeted oncolytic virotherapy that involves its establishment as a viable treatment option for cancer patients in the clinical setting.
Keywords: cell carrier; oncolytic virus; stem cell.
Figures

Similar articles
-
Crossing the boundaries: stem cells and gene therapy.Discov Med. 2010 Mar;9(46):192-6. Discov Med. 2010. PMID: 20350484 Free PMC article. Review.
-
Directing systemic oncolytic viral delivery to tumors via carrier cells.Cytokine Growth Factor Rev. 2010 Apr-Jun;21(2-3):119-26. doi: 10.1016/j.cytogfr.2010.02.004. Epub 2010 Mar 11. Cytokine Growth Factor Rev. 2010. PMID: 20226717 Free PMC article. Review.
-
Advanced new strategies for metastatic cancer treatment by therapeutic stem cells and oncolytic virotherapy.Oncotarget. 2016 Sep 6;7(36):58684-58695. doi: 10.18632/oncotarget.11017. Oncotarget. 2016. PMID: 27494901 Free PMC article. Review.
-
The utility of cells as vehicles for oncolytic virus therapies.Curr Opin Mol Ther. 2008 Aug;10(4):380-6. Curr Opin Mol Ther. 2008. PMID: 18683103 Review.
-
Employing RNA viruses to fight cancer: novel insights into oncolytic virotherapy.Biol Chem. 2017 Jul 26;398(8):891-909. doi: 10.1515/hsz-2017-0103. Biol Chem. 2017. PMID: 28441138 Review.
Cited by
-
Toll-like Receptor Signaling-deficient Cells Enhance Antitumor Activity of Cell-based Immunotherapy by Increasing Tumor Homing.Cancer Res Commun. 2023 Mar 1;3(3):347-360. doi: 10.1158/2767-9764.CRC-22-0365. eCollection 2023 Mar. Cancer Res Commun. 2023. PMID: 36875156 Free PMC article.
-
Targeting Cancer Stem Cells by Oncolytic Viruses and Nano-Mediated Delivery.Onco Targets Ther. 2020 Sep 22;13:9349-9350. doi: 10.2147/OTT.S279639. eCollection 2020. Onco Targets Ther. 2020. PMID: 33061422 Free PMC article. No abstract available.
-
Kinetics of Oncolytic Reovirus T3D Replication and Growth Pattern in Mesenchymal Stem Cells.Cell J. 2020 Oct;22(3):283-292. doi: 10.22074/cellj.2020.6686. Epub 2019 Dec 15. Cell J. 2020. PMID: 31863653 Free PMC article.
-
Metabolic signatures associated with oncolytic myxoma viral infections.Sci Rep. 2022 Jul 23;12(1):12599. doi: 10.1038/s41598-022-15562-3. Sci Rep. 2022. PMID: 35871072 Free PMC article.
-
Novel Chimeric Poxvirus CF17 Improves Survival in a Murine Model of Intraperitoneal Ovarian Cancer Metastasis.Mol Ther Oncolytics. 2020 Oct 10;19:278-282. doi: 10.1016/j.omto.2020.10.002. eCollection 2020 Dec 16. Mol Ther Oncolytics. 2020. PMID: 33251335 Free PMC article.
References
-
- Yang X., Chen E., Jiang H., Muszynski K., Harris R.D., Giardina S.L., Gromeier M., Mitra G., Soman G. Evaluation of ires-mediated, cell-type-specific cytotoxicity of poliovirus using a colorimetric cell proliferation assay. J. Virol. Methods. 2009;155:44–54. doi: 10.1016/j.jviromet.2008.09.020. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

