Association between Thiopurine S-Methyltransferase Polymorphisms and Azathioprine-Induced Adverse Drug Reactions in Patients with Autoimmune Diseases: A Meta-Analysis
- PMID: 26633017
- PMCID: PMC4669175
- DOI: 10.1371/journal.pone.0144234
Association between Thiopurine S-Methyltransferase Polymorphisms and Azathioprine-Induced Adverse Drug Reactions in Patients with Autoimmune Diseases: A Meta-Analysis
Abstract
Purpose: Azathioprine (AZA) is widely used as an immunosuppressive drug in autoimmune diseases, but its use is limited by significant adverse drug reactions (ADRs). Thiopurine S-methyltransferase (TPMT) is an important enzyme involved in AZA metabolism. Several clinical guidelines recommend determining TPMT genotype or phenotype before initiating AZA therapy. Although several studies have investigated the association between TPMT polymorphisms and AZA-induced ADRs, the results are inconsistent. The purpose of this study is to evaluate whether there is an association between TPMT polymorphisms and AZA-induced ADRs using meta-analysis.
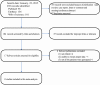
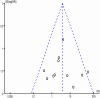
Methods: We explored PubMed, Web of Science and Embase for articles on TPMT polymorphisms and AZA-induced ADRs. Studies that compared TPMT polymorphisms with-ADRs and without-ADRs in patients with autoimmune diseases were included. Relevant outcome data from all the included articles were extracted and the pooled odds ratios (ORs) with corresponding 95% confidence intervals (CIs) were calculated using Revman 5.3 software.
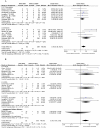
Results: Eleven published studies, with a total of 651 patients with autoimmune diseases, investigated associations between TPMT polymorphisms and AZA-induced ADRs, were included in this meta-analysis. Our meta-analysis demonstrated that TPMT polymorphisms were significantly associated with AZA-induced overall ADRs, bone marrow toxicity and gastric intolerance; pooled ORs were 3.12 (1.48-6.56), 3.76 (1.97-7.17) and 6.43 (2.04-20.25), respectively. TPMT polymorphisms were not associated with the development of hepatotoxicity; the corresponding pooled OR was 2.86 (95%CI: 0.32-25.86). However, the association in GI subset could be driven by one single study. After this study was excluded, the OR was 2.11 (95%CI: 0.36-12.42); namely, the association became negative.
Conclusions: Our meta-analysis demonstrated an association of TPMT polymorphisms with overall AZA-induced ADRs, bone marrow toxicity and gastric intolerance, but not with hepatotoxicity. The presence of the normal TPMT genotypes cannot preclude the development of ADRs during AZA treatment, TPMT genotyping prior to commencing AZA therapy cannot replace, may augment, the current practice of regular monitoring of the white blood cell. Because of small sample sizes, large and extensive exploration was required to validate our findings.
Conflict of interest statement
Figures

Similar articles
-
Association between thiopurine S-methyltransferase polymorphisms and thiopurine-induced adverse drug reactions in patients with inflammatory bowel disease: a meta-analysis.PLoS One. 2015 Mar 23;10(3):e0121745. doi: 10.1371/journal.pone.0121745. eCollection 2015. PLoS One. 2015. PMID: 25799415 Free PMC article.
-
The impact of thiopurine-S-methyltransferase genotype on the adverse drug reactions to azathioprine in patients with inflammatory bowel diseases.Bratisl Lek Listy. 2013;114(4):199-205. doi: 10.4149/bll_2013_042. Bratisl Lek Listy. 2013. PMID: 23514552
-
Thiopurine S-methyltransferase polymorphisms and thiopurine toxicity in treatment of inflammatory bowel disease.World J Gastroenterol. 2010 Jul 7;16(25):3187-95. doi: 10.3748/wjg.v16.i25.3187. World J Gastroenterol. 2010. PMID: 20593505 Free PMC article. Review.
-
Combined evaluation of genotype and phenotype of thiopurine S-methyltransferase (TPMT) in the clinical management of patients in chronic therapy with azathioprine.Drug Metab Pers Ther. 2019 Mar 6;34(1). doi: 10.1515/dmpt-2018-0037. Drug Metab Pers Ther. 2019. PMID: 30840585
-
Thiopurine methyltransferase: should it be measured before commencing thiopurine drug therapy?Ann Clin Biochem. 2004 Jul;41(Pt 4):294-302. doi: 10.1258/0004563041201455. Ann Clin Biochem. 2004. PMID: 15298741 Review.
Cited by
-
Genomic Profiling for Predictive Treatment Strategies in Fibrotic Interstitial Lung Disease.Biomedicines. 2024 Jun 21;12(7):1384. doi: 10.3390/biomedicines12071384. Biomedicines. 2024. PMID: 39061958 Free PMC article. Review.
-
Thiopurine S-methyltransferase polymorphisms in acute lymphoblastic leukemia, inflammatory bowel disease and autoimmune disorders: influence on treatment response.Pharmgenomics Pers Med. 2017 May 5;10:143-156. doi: 10.2147/PGPM.S108123. eCollection 2017. Pharmgenomics Pers Med. 2017. PMID: 28507448 Free PMC article. Review.
-
Preclinical evaluation of NUDT15-guided thiopurine therapy and its effects on toxicity and antileukemic efficacy.Blood. 2018 May 31;131(22):2466-2474. doi: 10.1182/blood-2017-11-815506. Epub 2018 Mar 23. Blood. 2018. PMID: 29572377 Free PMC article.
-
Tests that now deserve to be more widely adopted in IBD clinical practice.Therap Adv Gastroenterol. 2020 Jul 27;13:1756284820944088. doi: 10.1177/1756284820944088. eCollection 2020. Therap Adv Gastroenterol. 2020. PMID: 32782481 Free PMC article. Review.
-
Thiopurine Drugs in the Treatment of Ulcerative Colitis: Identification of a Novel Deleterious Mutation in TPMT.Genes (Basel). 2020 Oct 16;11(10):1212. doi: 10.3390/genes11101212. Genes (Basel). 2020. PMID: 33081236 Free PMC article. Review.
References
-
- Sinha AA, Lopez MT, McDevitt HO. Autoimmune diseases: the failure of self tolerance. Science. 1990;248(4961):1380–1388. - PubMed
-
- Goding JW. Autoimmune diseases. N Engl J Med. 2001;345(23):1707–1708. - PubMed
-
- Lennard L. The clinical pharmacology of 6-mercaptopurine. Eur J Clin Pharmacol. 1992;43(4):329–339. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

