PTEN deficiency reprogrammes human neural stem cells towards a glioblastoma stem cell-like phenotype
- PMID: 26632666
- PMCID: PMC4686761
- DOI: 10.1038/ncomms10068
PTEN deficiency reprogrammes human neural stem cells towards a glioblastoma stem cell-like phenotype
Abstract
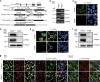
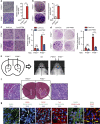
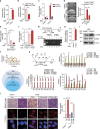
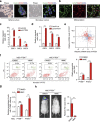
PTEN is a tumour suppressor frequently mutated in many types of cancers. Here we show that targeted disruption of PTEN leads to neoplastic transformation of human neural stem cells (NSCs), but not mesenchymal stem cells. PTEN-deficient NSCs display neoplasm-associated metabolic and gene expression profiles and generate intracranial tumours in immunodeficient mice. PTEN is localized to the nucleus in NSCs, binds to the PAX7 promoter through association with cAMP responsive element binding protein 1 (CREB)/CREB binding protein (CBP) and inhibits PAX7 transcription. PTEN deficiency leads to the upregulation of PAX7, which in turn promotes oncogenic transformation of NSCs and instates 'aggressiveness' in human glioblastoma stem cells. In a large clinical database, we find increased PAX7 levels in PTEN-deficient glioblastoma. Furthermore, we identify that mitomycin C selectively triggers apoptosis in NSCs with PTEN deficiency. Together, we uncover a potential mechanism of how PTEN safeguards NSCs, and establish a cellular platform to identify factors involved in NSC transformation, potentially permitting personalized treatment of glioblastoma.
Figures

Similar articles
-
Pten and p53 converge on c-Myc to control differentiation, self-renewal, and transformation of normal and neoplastic stem cells in glioblastoma.Cold Spring Harb Symp Quant Biol. 2008;73:427-37. doi: 10.1101/sqb.2008.73.047. Epub 2009 Jan 15. Cold Spring Harb Symp Quant Biol. 2008. PMID: 19150964
-
p53 and Pten control neural and glioma stem/progenitor cell renewal and differentiation.Nature. 2008 Oct 23;455(7216):1129-33. doi: 10.1038/nature07443. Nature. 2008. PMID: 18948956 Free PMC article.
-
Differential SKIP expression in PTEN-deficient glioblastoma regulates cellular proliferation and migration.Oncogene. 2015 Jul;34(28):3711-27. doi: 10.1038/onc.2014.303. Epub 2014 Sep 22. Oncogene. 2015. PMID: 25241900
-
PTEN expression and function in adult cancer stem cells and prospects for therapeutic targeting.Adv Biol Regul. 2014 Sep;56:66-80. doi: 10.1016/j.jbior.2014.07.002. Epub 2014 Jul 19. Adv Biol Regul. 2014. PMID: 25088603 Review.
-
Self-renewal mechanisms in neural cancer stem cells.Front Biosci (Landmark Ed). 2011 Jan 1;16(2):598-607. doi: 10.2741/3708. Front Biosci (Landmark Ed). 2011. PMID: 21196191 Review.
Cited by
-
Neural Stem Cells of the Subventricular Zone as the Origin of Human Glioblastoma Stem Cells. Therapeutic Implications.Front Oncol. 2019 Aug 20;9:779. doi: 10.3389/fonc.2019.00779. eCollection 2019. Front Oncol. 2019. PMID: 31482066 Free PMC article. Review.
-
Cellular rejuvenation: molecular mechanisms and potential therapeutic interventions for diseases.Signal Transduct Target Ther. 2023 Mar 14;8(1):116. doi: 10.1038/s41392-023-01343-5. Signal Transduct Target Ther. 2023. PMID: 36918530 Free PMC article. Review.
-
Modeling brain and neural crest neoplasms with human pluripotent stem cells.Neuro Oncol. 2023 Jul 6;25(7):1225-1235. doi: 10.1093/neuonc/noad034. Neuro Oncol. 2023. PMID: 36757217 Free PMC article. Review.
-
Repression of the Antioxidant NRF2 Pathway in Premature Aging.Cell. 2016 Jun 2;165(6):1361-1374. doi: 10.1016/j.cell.2016.05.017. Cell. 2016. PMID: 27259148 Free PMC article.
-
The landscape of aging.Sci China Life Sci. 2022 Dec;65(12):2354-2454. doi: 10.1007/s11427-022-2161-3. Epub 2022 Sep 2. Sci China Life Sci. 2022. PMID: 36066811 Free PMC article. Review.
References
-
- Furnari F. B. et al.. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 21, 2683–2710 (2007). - PubMed
-
- Bianco R. et al.. Loss of PTEN/MMAC1/TEP in EGF receptor-expressing tumor cells counteracts the antitumor action of EGFR tyrosine kinase inhibitors. Oncogene 22, 2812–2822 (2003). - PubMed
-
- Mellinghoff I. K. et al.. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N. Engl. J. Med. 353, 2012–2024 (2005). - PubMed
-
- Nagata Y. et al.. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer cell 6, 117–127 (2004). - PubMed
-
- Dean M., Fojo T. & Bates S. Tumour stem cells and drug resistance. Nat. Rev. Cancer. 5, 275–284 (2005). - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- SRA/SRP048488
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

