Alternative lipid emulsions versus pure soy oil based lipid emulsions for parenterally fed preterm infants
- PMID: 26630252
- PMCID: PMC7195185
- DOI: 10.1002/14651858.CD009172.pub2
Alternative lipid emulsions versus pure soy oil based lipid emulsions for parenterally fed preterm infants
Abstract
Background: The pure soybean oil based lipid emulsions (S-LE) conventionally used for parenteral nutrition (PN) in preterm infants have high polyunsaturated fatty acid (PUFA) content. The newer lipid emulsions (LE) from alternative lipid sources with reduced PUFA content may improve clinical outcomes in preterm infants.
Objectives: To determine the safety and efficacy of the newer alternative LE compared with the conventional S-LE for PN in preterm infants.
Search methods: We used the standard search strategy of the Cochrane Neonatal Review Group (CNRG) to search the Cochrane Central Register of Controlled Trials (CENTRAL; Issue 7), MEDLINE (1946 to 31 July 2015), EMBASE (1947 to 31 July 2015), CINAHL (1982 to 31 July 2015), Web of Science (31 July 2015), conference proceedings, trial registries (clinicaltrials.gov, controlled-trials.com, WHO's ICTRP), and the reference lists of retrieved articles for randomised controlled trials and quasi-randomised trials.
Selection criteria: Randomised or quasi-randomised controlled trials in preterm infants (< 37 weeks), comparing newer alternative LE with S-LE.
Data collection and analysis: Data collection and analysis conformed to the methods of the CNRG. We assessed the quality of evidence for important outcomes using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, in addition to reporting the conventional statistical significance of results.
Main results: Fifteen studies (N = 979 infants) are included in this review. Alternative LE including medium chain triglycerides/long chain triglycerides (MCT/LCT) LE (3 studies; n = 108), MCT-olive-fish-soy oil-LE (MOFS-LE; 7 studies; n = 469), MCT-fish-soy oil-LE (MFS-LE; 1 study; n = 60), olive-soy oil-LE (OS-LE; 7 studies; n = 406), and borage-soy oil-LE (BS-LE; 1 study; n = 34) were compared with S-LE. The different LE were also considered together to compare 'all fish oil containing-LE' versus S-LE (7 studies; n = 499) and 'all alternative LE' versus S-LE (15 studies; n = 979). Some studies had multiple intervention arms and were included in more than one comparison. No study compared pure fish oil-LE or structured-LE to S-LE.The GRADE quality of evidence (GRADE QoE) ranged from 'low' to 'very low.' Evidence came mostly from small single centre studies, many focusing on biochemical aspects as their primary outcomes, with optimal information size not achieved for the important clinical outcomes in any comparison.In the primary outcomes of the review there was a pooled effect towards decreased bronchopulmonary dysplasia (BPD) in OS-LE vs S-LE (4 studies, n = 261) not reaching statistical significance (typical risk ratio (RR) 0.69, 95% confidence interval (CI) 0.46 to 1.04, I² = 32%; typical risk difference (RD) -0.08, 95% CI -0.17 to 0.00, I² = 76%; GRADE QoE: 'very low'). No difference in BPD was observed in any other comparison. There were no statistically significant differences in the primary outcomes of death, growth rate (g/kg/day) or days to regain birth weight in any comparison.Retinopathy of prematurity (ROP) stage 1-2 was reported to be statistically significantly lower in one single centre study (n = 80) in the MOFS-LE group compared with the S-LE group (1/40 vs 12/40, respectively; RR 0.08, 95% CI 0.01 to 0.61; RD -0.27, 95% CI -0.43 to -0.12; number needed to benefit (NNTB) 4, 95% CI 2 to 8). However there were no statistically significant differences in the secondary outcome of ROP ≥ stage 3 in any of the individual studies or in any comparison (GRADE QoE: 'low' to 'very low'). No other study reported on ROP stages 1 and 2 separately.There were no statistically significant differences in the secondary outcomes of sepsis, PN associated liver disease (PNALD)/cholestasis, ventilation duration, necrotising enterocolitis (NEC) ≥ stage 2, jaundice requiring treatment, intraventricular haemorrhage grade III-IV, periventricular leukomalacia (PVL), patent ductus arteriosus (PDA), hypertriglyceridaemia, and hyperglycaemia in any comparison.No study reported on neurodevelopmental outcomes or essential fatty acid deficiency.
Authors' conclusions: All lipid emulsions in this review appeared to be safe and were well tolerated in preterm infants. Compared with the pure soy oil based LE, use of MOFS-LE was associated with a decrease in the early stages (1-2) of ROP in one study. However there were no statistically significant differences in clinically important outcomes including death, growth, BPD, sepsis, ROP ≥ stage 3, and PNALD with the use of newer alternative LE versus the conventional pure soy oil based LE (GRADE QoE ranged from 'low' to 'very low'). Currently there is insufficient evidence to recommend any alternative LE over S-LE or vice versa in preterm infants.Larger randomised studies focusing on important clinical outcomes, targeting specific 'at risk' population subgroups (e.g. extreme prematurity, long term PN, etc), and exploring the effect of different proportions of lipid constituents are required to evaluate the effectiveness of newer lipid emulsions compared with the conventional pure soy based LE in preterm infants.
Conflict of interest statement
None.
Figures
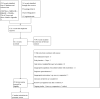
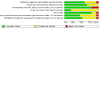






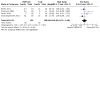












































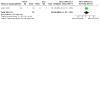
















































Update of
- doi: 10.1002/14651858.CD009172
Similar articles
-
Lipid emulsions for parenterally fed preterm infants.Cochrane Database Syst Rev. 2019 Jun 4;6(6):CD013163. doi: 10.1002/14651858.CD013163.pub2. Cochrane Database Syst Rev. 2019. PMID: 31158919 Free PMC article.
-
Lipid emulsions for parenterally fed term and late preterm infants.Cochrane Database Syst Rev. 2019 Jun 4;6(6):CD013171. doi: 10.1002/14651858.CD013171.pub2. Cochrane Database Syst Rev. 2019. PMID: 31158920 Free PMC article.
-
Inositol in preterm infants at risk for or having respiratory distress syndrome.Cochrane Database Syst Rev. 2019 Jul 8;7(7):CD000366. doi: 10.1002/14651858.CD000366.pub4. Cochrane Database Syst Rev. 2019. PMID: 31283839 Free PMC article.
-
Early introduction of lipids to parenterally-fed preterm infants.Cochrane Database Syst Rev. 2005 Apr 18;(2):CD005256. doi: 10.1002/14651858.CD005256. Cochrane Database Syst Rev. 2005. PMID: 15846747 Review.
-
Early erythropoiesis-stimulating agents in preterm or low birth weight infants.Cochrane Database Syst Rev. 2017 Nov 16;11(11):CD004863. doi: 10.1002/14651858.CD004863.pub5. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Feb 11;2:CD004863. doi: 10.1002/14651858.CD004863.pub6 PMID: 29145693 Free PMC article. Updated. Review.
Cited by
-
Emerging Clinical Benefits of New-Generation Fat Emulsions in Preterm Neonates.Nutr Clin Pract. 2017 Jun;32(3):326-336. doi: 10.1177/0884533616687500. Epub 2017 Jan 27. Nutr Clin Pract. 2017. PMID: 28129045 Free PMC article. Review.
-
A More Comprehensive Approach to the Neuroprotective Potential of Long-Chain Polyunsaturated Fatty Acids in Preterm Infants Is Needed-Should We Consider Maternal Diet and the n-6:n-3 Fatty Acid Ratio?Front Pediatr. 2020 Jan 10;7:533. doi: 10.3389/fped.2019.00533. eCollection 2019. Front Pediatr. 2020. PMID: 31998669 Free PMC article. Review.
-
A Mixed Lipid Emulsion for Prevention of Parenteral Nutrition Associated Cholestasis in Extremely Low Birth Weight Infants: A Randomized Clinical Trial.J Pediatr. 2018 Mar;194:87-93.e1. doi: 10.1016/j.jpeds.2017.11.012. Epub 2017 Dec 18. J Pediatr. 2018. PMID: 29269199 Free PMC article. Clinical Trial.
-
Docosahexaenoic Acid and Arachidonic Acid Levels Are Associated with Early Systemic Inflammation in Extremely Preterm Infants.Nutrients. 2020 Jul 5;12(7):1996. doi: 10.3390/nu12071996. Nutrients. 2020. PMID: 32635612 Free PMC article.
-
Effects of Fish Oil (SMOFlipid®) and Olive Oil Lipid (ClinOleic®) on Neonatal Morbidities in Preterm Infants.Medeni Med J. 2022 Sep 21;37(3):240-247. doi: 10.4274/MMJ.galenos.2022.83548. Medeni Med J. 2022. PMID: 36128742 Free PMC article.
References
References to studies included in this review
Beken 2014 {published data only}
-
- Beken S, Dilli D, Fettah ND, Kabatas EU, Zenciroqlu A, Okumus N. The influence of fish-oil lipid emulsions on retinopathy of prematurity in very low birth weight infants: a randomized controlled trial. Early Human Development 2014;90(1):27-31. [PMID: ] - PubMed
D'ascenzo 2014 {published data only}
-
- D'Ascenzo R, Savini S, Biagetti C, Bellagamba MP, Marchionni P, Pompilio A, et al. Higher Docosahexaenoic acid, lower Arachidonic acid and reduced lipid tolerance with high doses of a lipid emulsion containing 15% fish oil: A randomized clinical trial. Clinical Nutrition 2014;33(6):1002-9. - PubMed
Demirel 2011 {published data only (unpublished sought but not used)}
-
- Demirel G, Oguz SS, Celik IH, Erdeve O, Uras N, Dilmen U. The metabolic effects of two different lipid emulsions used in parenterally fed premature infants--a randomised comparative study. Early Human Development 2012;88(7):499-501. [PMID: ] - PubMed
Deshpande 2009 {published data only (unpublished sought but not used)}
-
- Deshpande GC, Simmer K, Mori T, Croft K. Parenteral lipid emulsions based on olive oil compared with soybean oil in preterm (<28 weeks' gestation) neonates: a randomised controlled trial. Journal of Pediatric Gastroenterology and Nutrition 2009;49(5):619-25. [PMID: ] - PubMed
Gawecka 2008 {published data only (unpublished sought but not used)}
-
- Gawecka A, Michalkiewicz J, Kornacka MK, Luckiewicz B, Kubiszewska I. Immunologic properties differ in preterm infants fed olive oil vs soy-based lipid emulsions during parenteral nutrition. JPEN. Journal of Parenteral and Enteral Nutrition 2008;32(4):448-53. [PMID: ] - PubMed
Gobel 2003 {published data only}
-
- Göbel Y, Koletzko B, Böhles HJ, Engelsberger I, Forget D, Le Brun A, et al. Parenteral fat emulsions based on olive and soybean oils: a randomised clinical trial in preterm infants. Journal of Pediatric Gastroenterology and Nutrition 2003;37(2):161-7. [PMID: ] - PubMed
Koksal 2011 {published and unpublished data}
-
- Köksal N, Kavurt AV, Cetinkaya M, Ozarda Y, Ozkan H. Comparison of lipid emulsions on antioxidant capacity in preterm infants receiving parenteral nutrition. Pediatrics International 2011;53(4):562-6. [PMID: ] - PubMed
Lehner 2006 {published data only (unpublished sought but not used)}
-
- Lehner F, Demmelmair H, Röschinger W, Decsi T, Szász M, Adamovich K, et al. Metabolic effects of intravenous LCT or MCT/LCT lipid emulsions in preterm infants. Journal of Lipid Research 2006;47(2):404-11. [PMID: ] - PubMed
Rayyan 2012 {published data only (unpublished sought but not used)}
-
- Rayyan M, Devlieger H, Jochum F, Allegaert K. Short-term use of parenteral nutrition with a lipid emulsion containing a mixture of soybean oil, olive oil, medium-chain triglycerides, and fish oil: a randomised double-blind study in preterm infants. Journal of Parenteral and Enteral Nutrition 2012;36(1 Suppl):81S-94S. [PMID: ] - PMC - PubMed
Rubin 1994 {published data only}
-
- Rubin M, Moser A, Naor N, Merlob P, Pakula R, Sirota L. Effect of three intravenously administered fat emulsions containing different concentrations of fatty acids on the plasma fatty acid composition of premature infants. The Journal of Pediatrics 1994;125(4):596-602. [PMID: ] - PubMed
Savini 2013 {published data only (unpublished sought but not used)}
-
- Savini S, D'Ascenzo R, Biagetti C, Serpentini G, Pompilio A, Bartoli A, et al. The effect of 5 intravenous lipid emulsions on plasma phytosterols in preterm infants receiving parenteral nutrition: a randomised clinical trial. American Journal of Clinical Nutrition 2013;98(2):312-8. [PMID: ] - PubMed
Skouroliakou 2010 {published data only (unpublished sought but not used)}
-
- Skouroliakou M, Konstantinou D, Koutri K, Kakavelaki C, Stathopoulou M, Antoniadi M, et al. A double-blind, randomised clinical trial of the effect of omega-3 fatty acids on the oxidative stress of preterm neonates fed through parenteral nutrition. European Journal of Clinical Nutrition 2010;64(9):940-7. [PMID: ] - PubMed
Tomsits 2010 {published data only (unpublished sought but not used)}
-
- Tomsits E, Pataki M, Tölgyesi A, Fekete G, Rischak K, Szollár L. Safety and efficacy of a lipid emulsion containing a mixture of soybean oil, medium-chain triglycerides, olive oil, and fish oil: a randomised, double-blind clinical trial in premature infants requiring parenteral nutrition. Journal of Pediatric Gastroenterology and Nutrition 2010;51(4):514-21. [PMID: ] - PubMed
Vlaardingerbroek 2014 {published data only}
-
- Vlaardingerbroek H, Vermeulen MJ, Carnielli VP, Vaz FM, den Akker CH, Goudoever JB. Growth and fatty acid profiles of VLBW infants receiving a multicomponent lipid emulsion from birth. Journal of Pediatric Gastroenterology and Nutrition 2014;58(4):417-27. [PMID: ] - PubMed
Wang 2015 {published data only}
-
- Wang Y, Zhou KJ, Tang QY, Hong L, Feng Y, Lu LN, et al. Effect of an Olive Oil-Based Lipid Emulsion Compared With a Soybean Oil-Based Lipid Emulsion on Liver Chemistry and Bile Acid Composition in Preterm Infants Receiving Parenteral Nutrition: A Double-Blind, Randomized Trial. Journal of Parenteral and Enteral Nutrition 2015 Jan 5 [Epub ahead of print]. [PMID: ] - PubMed
References to studies excluded from this review
Angsten 2002 {published data only}
-
- Angsten G, Boberg M, Cederblad G, Meurling S, Stiernström H. Metabolic effects in neonates receiving intravenous medium-chain triglycerides. Acta Paediatrica 2002;91(2):188-97. [PMID: ] - PubMed
Ariyawangso {published data only}
-
- Ariyawangso U, Puttilerpong C, Ratanachuek S, Anuntkosol M. Short-term safety and efficacy of fish-oil emulsions on the prevention of parenteral nutrition-associated liver disease in surgical neonates: a randomized controlled trial. Thai Journal of Pharmaceutical Sciences 2014;38(4):202-9.
Lam 2014 {published data only}
-
- Lam HS, Tam YH, Poon TC, Cheung HM, Yu X, Chan BP, et al. A double-blind randomised controlled trial of fish oil-based versus soy-based lipid preparations in the treatment of infants with parenteral nutrition-associated cholestasis. Neonatology 2014;105(4):290-6. [PMID: ] - PubMed
Lima 1988 {published data only}
-
- Lima LA, Murphy JF, Stansbie D, Rowlandson P, Gray OP. Neonatal parenteral nutrition with a fat emulsion containing medium chain triglycerides. Acta Paediatrica Scandinavica 1988;77(3):332-9. [PMID: ] - PubMed
Magnusson 1997 {published data only}
-
- Magnusson G, Boberg M, Cederblad G, Meurling S. Plasma and tissue levels of lipids, fatty acids and plasma carnitine in neonates receiving a new fat emulsion. Acta Paediatrica 1997;86(6):638-44. [PMID: ] - PubMed
Nehra 2014 {published data only}
Webb 2008 {published data only}
-
- Webb AN, Hardy P, Peterkin M, Lee O, Shalley H, Croft KD, et al. Tolerability and safety of olive oil-based lipid emulsion in critically ill neonates: a blinded randomised trial. Nutrition 2008;24(11-12):1057-64. [PMID: ] - PubMed
Wilson 1997 {published data only}
References to ongoing studies
NCT01585935 {published data only}
-
- NCT01585935. Preventing Cholestasis Using SMOFLipid®. https://clinicaltrials.gov/ct2/show/NCT01585935 (accessed 17 August 2015).
NCT01683162 {published data only}
-
- NCT01683162. Effects of Parenteral Nutrition With Different Lipid Emulsions in Preterm Infants. https://clinicaltrials.gov/ct2/show/NCT01683162.
Additional references
Beauchamp 2005
-
- Beauchamp GK, Keast RS, Morel D, Lin J, Pika J, Han Q, et al. Phytochemistry: ibuprofen-like activity in extra-virgin olive oil. Nature 2005;437(7055):45-6. [PMID: ] - PubMed
Bell 1978
Buenestado 2006
-
- Buenestado A, Cortijo J, Sanz MJ, Naim-Abu-Nabah Y, Martinez-Losa M, Mata M, et al. Olive oil-based lipid emulsion's neutral effects on neutrophil functions and leukocyte-endothelial cell interactions. Journal of Parenteral and Enteral Nutrition 2006;30(4):286-96. [PMID: ] - PubMed
Christensen 2007
-
- Christensen RD, Henry E, Wiedmeier SE, Burnett J, Lambert DK. Identifying patients, on the first day of life, at high-risk of developing parenteral nutrition-associated liver disease. Journal of Perinatology 2007;27(5):284-90. [PMID: ] - PubMed
Cober 2010
-
- Cober MP, Teitelbaum DH. Prevention of parenteral nutrition-associated liver disease: lipid minimization. Current Opinion in Organ Transplantation 2010;15(3):330-3. [PMID: ] - PubMed
de Meijer 2009
-
- Meijer VE, Gura KM, Le HD, Meisel JA, Puder M. Fish oil-based lipid emulsions prevent and reverse parenteral nutrition-associated liver disease: the Boston experience. Journal of Parenteral and Enteral Nutrition 2009;33(5):541-7. - PubMed
de Vries 1992
-
- Vries LS, Eken P, Dubowitz LM. The spectrum of leucomalacia using cranial ultrasound. Behavioural Brain Research 1992;49(1):1-6. [PMID: ] - PubMed
Deeks 2011
-
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Driscoll 2008
-
- Driscoll DF, Bistrian BR, Demmelmair H, Koletzko B. Pharmaceutical and clinical aspects of parenteral lipid emulsions in neonatology. Clinical Nutrition 2008;27(4):497-503. [PMID: ] - PubMed
EndNote 2014 [Computer program]
-
- EndNote. Version 6.0.1. Thompson Reuters, 2014.
Furukawa 2006
-
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta-analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(1):7-10. [PMID: ] - PubMed
Fürst 2000
-
- Fürst P, Kuhn KS. Fish oil emulsions: what benefits can they bring? Clinical Nutrition 2000;19(1):7-14. [PMID: ] - PubMed
Gawecka 2008a
-
- Gawecka A, Michalkiewicz J, Kornacka MK, Luckiewicz B, Kubiszewska I. Immunologic properties differ in preterm infants fed olive oil vs soy-based lipid emulsions during parenteral nutrition. Journal of Parenteral and Enteral Nutrition 2008;32(4):448-53. [PMID: ] - PubMed
Gawecka 2008b
-
- Gawecka A, Kornacka MK, Luckiewicz B, Rudzinska I. Tolerance of two lipid emulsions used in parenterally-fed premature infants - a comparative study. Medycyna Wieku Rozwojowego 2008;12(3):782-8. [PMID: ] - PubMed
Gitto 2001
-
- Gitto E, Karbownik M, Reiter RJ, Tan DX, Cuzzocrea S, Chiurazzi P, et al. Effects of melatonin treatment in septic newborns. Pediatric Research 2001;50(6):756-60. [PMID: ] - PubMed
Gogos 1995
-
- Gogos CA, Kalfarentzos F. Total parenteral nutrition and immune system activity: a review. Nutrition 1995;11(4):339-44. [PMID: ] - PubMed
Goulet 1999
-
- Goulet O, Potter S, Antebi H, Driss F, Colomb V, Bereziat G, et al. Long-term efficacy and safety of a new olive oil-based intravenous fat emulsion in paediatric patients: a double-blind randomised study. American Journal of Clinical Nutrition 1999;70(3):338-45. [PMID: ] - PubMed
GradePro 2008 [Computer program]
-
- McMaster University, 2008 GradePro Version 3.2. McMaster University, 2008.
Granato 2000
-
- Granato D, Blum S, Rossle C, Le Boucher J, Malnoe A, Dutot G. Effects of parenteral lipid emulsions with different fatty acid composition on immune cell functions in vitro. Journal of Parenteral and Enteral Nutrition 2000;24(2):113-8. [PMID: ] - PubMed
Grimm 2005
-
- Grimm H. A balanced lipid emulsion - a new concept in parenteral nutrition. Clinical Nutrition Supplements 2005;1(3):25-30.
Gura 2005
-
- Gura KM, Parsons SK, Bechard LJ, Henderson T, Dorsey M, Phipatanakul W, et al. Use of a fish oil-based lipid emulsion to treat essential fatty acid deficiency in a soy allergic patient receiving parenteral nutrition. Clinical nutrition 2005;24(5):839-47. [PMID: ] - PubMed
Guyatt 2008
Guyatt 2011
-
- Guyatt G, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, et al. GRADE guidelines: 6. Rating the quality of evidence--imprecision. Journal of Clinical Epidemiology 2011;64(12):1283-93. [PMID: ] - PubMed
Guyatt 2011a
-
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [PMID: ] - PubMed
Guyatt 2011b
-
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso-Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence--study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64(4):407-15. [PMID: ] - PubMed
Guyatt 2011c
-
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence--inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294-302. [PMID: ] - PubMed
Guyatt 2011d
-
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence--indirectness. Journal of Clinical Epidemiology 2011;64(12):1303-10. [PMID: ] - PubMed
Haynes 2005
Heird 2005
-
- Heird WC, Lapillonne A. The role of essential fatty acids in development. Annual Review of Nutrition 2005;25:549-71. [PMID: ] - PubMed
Henriksen 2008
-
- Henriksen C, Haugholt K, Lindgren M, Aurvag AK, Ronnestad A, Gronn M, et al. Improved cognitive development among preterm infants attributable to early supplementation of human milk with docosahexaenoic acid and arachidonic acid. Pediatrics 2008;121(6):1137-45. [PMID: ] - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JPT, Deeks JJ. Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Hozo 2005
ICROP 2005
-
- International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Archives of Ophthalmology 2005;123(7):991-9. [PMID: ] - PubMed
Kapoor 2019
Koletzko 2001
-
- Koletzko B, Agostoni C, Carlson SE, Clandinin T, Hornstra G, Neuringer M, et al. Long chain polyunsaturated fatty acids (LC-PUFA) and perinatal development. Acta Paediatrica 2001;90(4):460-4. [PMID: ] - PubMed
Koletzko 2003
-
- Koletzko B, Sauerwald U, Keicher U, Saule H, Wawatschek S, Bohles H, et al. Fatty acid profiles, antioxidant status, and growth of preterm infants fed diets without or with long-chain polyunsaturated fatty acids. A randomized clinical trial. European Journal of Nutrition 2003;42(5):243-53. [PMID: ] - PubMed
Koletzko 2005
-
- Koletzko B, Goulet O, Hunt J, Krohn K, Shamir R. Guidelines on Paediatric Parenteral Nutrition of the European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and the European Society for Clinical Nutrition and Metabolism (ESPEN), supported by the European Society of Paediatric Research (ESPR). Journal of Pediatric Gastroenterology and Nutrition 2005;41(Suppl 2):S1-87. [PMID: ] - PubMed
Koletzko 2010
-
- Koletzko B, Goulet O. Fish oil containing intravenous lipid emulsions in parenteral nutrition-associated cholestatic liver disease. Current Opinion in Clinical Nutrition and Metabolic Care 2010 May;13(3):321-6. [PMID: ] - PubMed
Krohn 2006
-
- Krohn K, Koletzko B. Parenteral lipid emulsions in paediatrics. Current Opinion in Clinical Nutrition and Metabolic Care 2006;9(3):319-23. [PMID: ] - PubMed
Lapillonne 2013
-
- Lapillonne A, Groh-Wargo S, Gonzalez CH, Uauy R. Lipid needs of preterm infants: updated recommendations. The Journal of Pediatrics 2013;162(3 Suppl):S37-47. [PMID: ] - PubMed
Lekka 2004
-
- Lekka ME, Liokatis S, Nathanail C, Galani V, Nakos G. The impact of intravenous fat emulsion administration in acute lung injury. American Journal of Respiratory and Critical Care Medicine 2004;169(5):638-44. [PMID: ] - PubMed
McNaught 1997
-
- McNaught AD, Wilkinson A. IUPAC. Compendium of Chemical Terminology (the "Gold Book"); http://goldbook.iupac.org/P04758.html (2006-). 2nd edition. Oxford: Blackwell Scientific Publications, 1997.
Mylonas 1999
-
- Mylonas C, Kouretas D. Lipid peroxidation and tissue damage. In Vivo 1999;13(3):295-309. [PMID: ] - PubMed
Nandiwada 2013
-
- Nandivada P, Carlson SJ, Cowan E, Chang MI, Gura KM, Puder M. Role of parenteral lipid emulsions in the preterm infant. Early Human Development 2013;89(Suppl 2):S45-9. [PMID: ] - PubMed
Palmblad 1991
-
- Palmblad J. Intravenous lipid emulsions and host defence - a critical review. Clinical Nutrition 1991;10(6):303-8. [PMID: ] - PubMed
Papile 1978
-
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular haemorrhage: a study of infants with birth weights less than 1,500 gm. Journal of Pediatrics 1978;92(4):529-34. [PMID: ] - PubMed
Pawlik 2011
-
- Pawlik D, Lauterbach R, Turyk E. Fish-oil fat emulsion supplementation may reduce the risk of severe retinopathy in VLBW infants. Pediatrics 2011;127(2):223-8. [PMID: ] - PubMed
Pawlik 2013
-
- Pawlik D, Lauterbach R, Walczak M, Hurkala J, Sherman MP. Fish-Oil Fat Emulsion Supplementation Reduces the Risk of Retinopathy in Very Low Birth Weight Infants: A Prospective, Randomized Study. Journal of Parenteral and Enteral Nutrition 2013;38(6):711-6. [PMID: ] - PubMed
Pitkanen 1991
-
- Pitkanen O, Hallman M, Andersson S. Generation of free radicals in lipid emulsion used in parenteral nutrition. Pediatric Research 1991;29(1):56-9. [PMID: ] - PubMed
Puder 2009
Putet 2000
-
- Putet G. Lipid metabolism of the micropremie. Clinics in Perinatology 2000;27(1):57-69. [PMID: ] - PubMed
Reimund 2004
-
- Reimund JM, Scheer O, Muller CD, Pinna G, Duclos B, Baumann R. In vitro modulation of inflammatory cytokine production by three lipid emulsions with different fatty acid compositions. Clinical Nutrition 2004;23(6):1324-32. [PMID: ] - PubMed
RevMan 2011 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.0.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Robinson 2008
-
- Robinson DT, Ehrenkranz RA. Parenteral nutrition-associated cholestasis in small for gestational age infants. The Journal of Pediatrics 2008;152(1):59-62. [PMID: ] - PubMed
Rubin 1995
-
- Rubin M, Naor N, Sirota L, Moser A, Pakula R, Harell D, et al. Are bilirubin and plasma lipid profiles of premature infants dependent on the lipid emulsion infused? Journal of Pediatric Gastroenterology and Nutrition 1995;21(1):25-30. [PMID: ] - PubMed
Sala‐Vila 2007
-
- Sala-Vila A, Barbosa VM, Calder PC. Olive oil in parenteral nutrition. Current Opinion in Clinical Nutrition and Metabolic Care 2007;10(2):165-74. - PubMed
SanGiovanni 2000
-
- SanGiovanni JP, Berkey CS, Dwyer JT, Colditz GA. Dietary essential fatty acids, long-chain polyunsaturated fatty acids, and visual resolution acuity in healthy full term infants: a systematic review. Early Human Development 2000;57(3):165-88. [PMID: ] - PubMed
Schock 2001
-
- Schock BC, Sweet DG, Halliday HL, Young IS, Ennis M. Oxidative stress in lavage fluid of preterm infants at risk of chronic lung disease. American Journal of Physiology. Lung Cellular and Molecular Physiology 2001;281(6):L1386-91. [PMID: ] - PubMed
Sinclair 2009
Skouroliakou 2012
-
- Skouroliakou M, Konstantinou D, Agakidis C, Delikou N, Koutri K, Antoniadi M, et al. Cholestasis, bronchopulmonary dysplasia, and lipid profile in preterm infants receiving MCT/ω-3-PUFA-containing or soybean-based lipid emulsions. European Journal of Clinical Nutrition 2012 Dec;27(6):817-24. - PubMed
Smithers 2008
-
- Smithers LG, Gibson RA, McPhee A, Makrides M. Higher dose of docosahexaenoic acid in the neonatal period improves visual acuity of preterm infants: results of a randomized controlled trial. The American journal of clinical nutrition 2008;88(4):1049-56. [PMID: ] - PubMed
Sterne 2011
-
- Sterne JAC, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. In: Available from www.cochrane-handbook.org.
Stoll 2002
-
- Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics 2002;110(2 Pt 1):285-91. [PMID: ] - PubMed
Van Kempen 2006
-
- Kempen AA, Crabben SN, Ackermans MT, Endert E, Kok JH, Sauerwein HP. Stimulation of gluconeogenesis by intravenous lipids in preterm infants: response depends on fatty acid profile. American Journal of Physiology, Endocrinology and Metabolism 2006;290(4):E723-30. [PMID: ] - PubMed
Vanek 2012
-
- Vanek VW, Seidner DL, Allen P, Bistrian B, Collier S, Gura K, et al. A.S.P.E.N. position paper: Clinical role for alternative intravenous fat emulsions. Nutrition in Clinical Practice 2012;27(2):150-92. [PMID: ] - PubMed
Vlaardingerbroek 2012
-
- Vlaardingerbroek H, Veldhorst MA, Spronk S, den Akker CH, Goudoever JB. Parenteral lipid administration to very-low-birth-weight infants--early introduction of lipids and use of new lipid emulsions: a systematic review and meta-analysis. American Journal of Clinical Nutrition 2012;96(2):255-68. [PMID: ] - PubMed
Vlaardingerbroek 2013
-
- Vlaardingerbroek H, Vermeulen MJ, Rook D, den Akker CH, Dorst K, Wattimena JL, et al. Safety and efficacy of early parenteral lipid and high-dose amino acid administration to very low birth weight infants. The Journal of Pediatrics 2013;163(3):638-44.e1-5. [PMID: ] - PubMed
Waitzberg 2006
-
- Waitzberg DL, Torrinhas RS, Jacintho TM. New parenteral lipid emulsions for clinical use. JPEN. Journal of Parenteral and Enteral Nutrition 2006;30(4):351-67. [PMID: ] - PubMed
Walsh 2004
-
- Walsh MC, Yao Q, Gettner P, Hale E, Collins M, Hensman A, et al. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics 2004;114(5):1305-11. [PMID: ] - PubMed
Wanten 2007
-
- Wanten GJ, Calder PC. Immune modulation by parenteral lipid emulsions. American Journal of Clinical Nutrition 2007;85(5):1171-84. [PMID: ] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

