The effect of siRNA-mediated lymphocyte-specific protein tyrosine kinase (Lck) inhibition on pulmonary inflammation in a mouse model of asthma
- PMID: 26628998
- PMCID: PMC4658887
The effect of siRNA-mediated lymphocyte-specific protein tyrosine kinase (Lck) inhibition on pulmonary inflammation in a mouse model of asthma
Abstract
Objective: To explore the effect of siRNA-mediated inhibition of lymphocyte-specific protein tyrosine kinase (Lck) on pulmonary inflammation in a mouse model of asthma.
Methods: A total of 32 female BABL/c mice were used in the study. The mouse asthma model was established with ovabumin (OVA), and Lck specific siRNA or nonspecific siRNA was transfected through the tail vein before the first OVA challenge. Two days after the last challenge, mice were sacrificed and bronchoalveolar lavage fluid (BALF), plasma and lung tissue were collected. Levels of Lck mRNA and protein in lung were detected by quantitative real-time PCR and western blot. The levels of IL-4 and IgE in BALF and plasma were detected with ELISA.
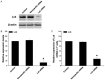
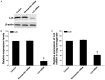
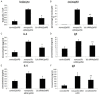
Results: Lck specific siRNA significantly inhibited expression of Lck mRNA and protein in T cells. In vivo transfection of Lck siRNA down regulated the expression of Lck mRNA and protein in lung parenchymal homogenates. Sensitized mice treated with Lck siRNA prior to OVA challenge had fewer eosinophils in BALF and in lung sections and lower levels of IL-4 and IgE in BALF and plasma compared to those treated with nonspecific siRNA.
Conclusions: Pretreatment of OVA sensitized mice with Lck siRNA results in attenuation of pulmonary inflammation following OVA challenge. Inhibition of Lck gene expression should be investigated further as a potential therapy for asthma.
Keywords: IL-4; IgE; T lymphocyte; in vivo transfection.
Figures


Similar articles
-
[Effects of fasudil on the expression of Rho kinase-1 and airway inflammation in a mouse model of asthma.].Zhonghua Jie He He Hu Xi Za Zhi. 2009 Nov;32(11):847-9. Zhonghua Jie He He Hu Xi Za Zhi. 2009. PMID: 20079297 Chinese.
-
Status of Stat3 in an ovalbumin-induced mouse model of asthma: analysis of the role of Socs3 and IL-6.Int Arch Allergy Immunol. 2009;148(2):99-108. doi: 10.1159/000155740. Epub 2008 Sep 18. Int Arch Allergy Immunol. 2009. PMID: 18799889
-
Effect of ageing on pulmonary inflammation, airway hyperresponsiveness and T and B cell responses in antigen-sensitized and -challenged mice.Clin Exp Allergy. 2007 Sep;37(9):1392-403. doi: 10.1111/j.1365-2222.2007.02775.x. Clin Exp Allergy. 2007. PMID: 17845421 Free PMC article.
-
Bu-Shen-Yi-Qi formulae suppress chronic airway inflammation and regulate Th17/Treg imbalance in the murine ovalbumin asthma model.J Ethnopharmacol. 2015 Apr 22;164:368-77. doi: 10.1016/j.jep.2015.01.016. Epub 2015 Jan 24. J Ethnopharmacol. 2015. PMID: 25625352
-
[Combined effects of neonatal Bacillus Calmette-Guerin vaccination and respiratory syncytial infection on experimental asthma in mice].Zhonghua Er Ke Za Zhi. 2006 Jun;44(6):420-4. Zhonghua Er Ke Za Zhi. 2006. PMID: 16836848 Chinese.
Cited by
-
Could the Epigenetics of Eosinophils in Asthma and Allergy Solve Parts of the Puzzle?Int J Mol Sci. 2021 Aug 19;22(16):8921. doi: 10.3390/ijms22168921. Int J Mol Sci. 2021. PMID: 34445627 Free PMC article. Review.
-
Comparing DNA methylation profiles across different tissues associated with the diagnosis of pediatric asthma.Sci Rep. 2020 Jan 13;10(1):151. doi: 10.1038/s41598-019-56310-4. Sci Rep. 2020. PMID: 31932625 Free PMC article.
-
Pharmacological targeting of allergen-specific T lymphocytes.Immunol Lett. 2017 Sep;189:27-39. doi: 10.1016/j.imlet.2017.03.010. Epub 2017 Mar 18. Immunol Lett. 2017. PMID: 28322861 Free PMC article. Review.
-
New horizons in drug discovery of lymphocyte-specific protein tyrosine kinase (Lck) inhibitors: a decade review (2011-2021) focussing on structure-activity relationship (SAR) and docking insights.J Enzyme Inhib Med Chem. 2021 Dec;36(1):1574-1602. doi: 10.1080/14756366.2021.1937143. J Enzyme Inhib Med Chem. 2021. PMID: 34233563 Free PMC article. Review.
-
Blockade of Tyrosine Kinase, LCK Leads to Reduction in Airway Inflammation through Regulation of Pulmonary Th2/Treg Balance and Oxidative Stress in Cockroach Extract-Induced Mouse Model of Allergic Asthma.Metabolites. 2022 Aug 25;12(9):793. doi: 10.3390/metabo12090793. Metabolites. 2022. PMID: 36144198 Free PMC article.
References
-
- Fitzgerald JM, Bateman E, Hurd S, Boulet LP, Haahtela T, Cruz AA, Levy ML. The GINA Asthma Challenge: reducing asthma hospitalisations. Eur Respir J. 2011;38:997–8. - PubMed
-
- Anderson GP. Endotyping asthma: new insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet. 2008;372:1107–19. - PubMed
-
- Robinson DS. The role of the T cell in asthma. J Allergy ClinImmunol. 2010;126:1081–91. quiz 1092-3. - PubMed
-
- Robinson DS. The role of the T cell in asthma. J Allergy ClinImmunol. 2010;126:1081–91. quiz 1092-3. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
