Delivering Transgenic DNA Exceeding the Carrying Capacity of AAV Vectors
- PMID: 26611576
- PMCID: PMC4971574
- DOI: 10.1007/978-1-4939-3271-9_2
Delivering Transgenic DNA Exceeding the Carrying Capacity of AAV Vectors
Abstract
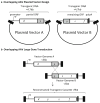
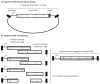
Gene delivery using recombinant adeno-associated virus (rAAV) has emerged to the forefront demonstrating safe and effective phenotypic correction of diverse diseases including hemophilia B and Leber's congenital amaurosis. In addition to rAAV's high efficiency of transduction and the capacity for long-term transgene expression, the safety profile of rAAV remains unsoiled in humans with no deleterious vector-related consequences observed thus far. Despite these favorable attributes, rAAV vectors have a major disadvantage preventing widespread therapeutic applications; as the AAV capsid is the smallest described to date, it cannot package "large" genomes. Currently, the packaging capacity of rAAV has yet to be definitively defined but is approximately 5 kb, which has served as a limitation for large gene transfer. There are two main approaches that have been developed to overcome this limitation, split AAV vectors, and fragment AAV (fAAV) genome reassembly (Hirsch et al., Mol Ther 18(1):6-8, 2010). Split rAAV vector applications were developed based upon the finding that rAAV genomes naturally concatemerize in the cell post-transduction and are substrates for enhanced homologous recombination (HR) (Hirsch et al., Mol Ther 18(1):6-8, 2010; Duan et al., J Virol 73(1):161-169, 1999; Duan et al., J Virol 72(11):8568-8577, 1998; Duan et al., Mol Ther 4(4):383-391, 2001; Halbert et al., Nat Biotechnol 20(7):697-701, 2002). This method involves "splitting" the large transgene into two separate vectors and upon co-transduction, intracellular large gene reconstruction via vector genome concatemerization occurs via HR or nonhomologous end joining (NHEJ). Within the split rAAV approaches there currently exist three strategies: overlapping, trans-splicing, and hybrid trans-splicing (Duan et al., Mol Ther 4(4):383-391, 2001; Halbert et al., Nat Biotechnol 20(7):697-701, 2002; Ghosh et al., Mol Ther 16(1):124-130, 2008; Ghosh et al., Mol Ther 15(4):750-755, 2007). The other major strategy for AAV-mediated large gene delivery is the use of fragment AAV (fAAV) (Dong et al., Mol Ther 18(1):87-92, 2010; Hirsch et al., Mol Ther 21(12):2205-2216, 2013; Lai et al., Mol Ther 18(1):75-79, 2010; Wu et al., Mol Ther 18(1):80-86, 2010). This strategy developed following the observation that the attempted encapsidation of transgenic cassettes exceeding the packaging capacity of the AAV capsid results in the packaging of heterogeneous single-strand genome fragments (<5 kb) of both polarities (Dong et al., Mol Ther 18(1):87-92, 2010; Hirsch et al., Mol Ther 21(12):2205-2216, 2013; Lai et al., Mol Ther 18(1):75-79, 2010; Wu et al., Mol Ther 18(1):80-86, 2010). After transduction by multiple fAAV particles, the genome fragments can undergo opposite strand annealing, followed by host-mediated DNA synthesis to reconstruct the intended oversized genome within the cell. Although, there appears to be growing debate as to the most efficient method of rAAV-mediated large gene delivery, it remains possible that additional factors including the target tissue and the transgenomic sequence factor into the selection of a particular approach for a specific application (Duan et al., Mol Ther 4(4):383-391, 2001; Ghosh et al., Mol Ther 16(1):124-130, 2008; Hirsch et al., Mol Ther 21(12):2205-2216, 2013; Trapani et al., EMBO Mol Med 6(2):194-211, 2014; Ghosh et al., Hum Gene Ther 22(1):77-83, 2011). Herein we discuss the design, production, and verification of the leading rAAV large gene delivery strategies.
Keywords: Adeno-associated virus; Concatemer; Fragment; Hybrid; Large gene delivery; Overlapping; Split AAV method; Trans-splicing; Vector capacity.
Figures

Similar articles
-
Evaluation of the fate of rAAV genomes following in vivo administration.Methods Mol Biol. 2011;807:239-58. doi: 10.1007/978-1-61779-370-7_10. Methods Mol Biol. 2011. PMID: 22034033
-
Expressing Transgenes That Exceed the Packaging Capacity of Adeno-Associated Virus Capsids.Hum Gene Ther Methods. 2016 Feb;27(1):1-12. doi: 10.1089/hgtb.2015.140. Hum Gene Ther Methods. 2016. PMID: 26757051 Free PMC article. Review.
-
AAV-mediated gene editing via double-strand break repair.Methods Mol Biol. 2014;1114:291-307. doi: 10.1007/978-1-62703-761-7_19. Methods Mol Biol. 2014. PMID: 24557911 Free PMC article.
-
Oversized AAV transductifon is mediated via a DNA-PKcs-independent, Rad51C-dependent repair pathway.Mol Ther. 2013 Dec;21(12):2205-16. doi: 10.1038/mt.2013.184. Epub 2013 Aug 13. Mol Ther. 2013. PMID: 23939025 Free PMC article.
-
Adeno-Associated Virus Engineering and Load Strategy for Tropism Modification, Immune Evasion and Enhanced Transgene Expression.Int J Nanomedicine. 2024 Jul 29;19:7691-7708. doi: 10.2147/IJN.S459905. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39099791 Free PMC article. Review.
Cited by
-
Genetically Modified Mesenchymal Stromal/Stem Cells: Application in Critical Illness.Stem Cell Rev Rep. 2020 Oct;16(5):812-827. doi: 10.1007/s12015-020-10000-1. Stem Cell Rev Rep. 2020. PMID: 32671645 Free PMC article. Review.
-
Transition from Animal-Based to Human Induced Pluripotent Stem Cells (iPSCs)-Based Models of Neurodevelopmental Disorders: Opportunities and Challenges.Cells. 2023 Feb 7;12(4):538. doi: 10.3390/cells12040538. Cells. 2023. PMID: 36831205 Free PMC article. Review.
-
New Editing Tools for Gene Therapy in Inherited Retinal Dystrophies.CRISPR J. 2022 Jun;5(3):377-388. doi: 10.1089/crispr.2021.0141. Epub 2022 May 3. CRISPR J. 2022. PMID: 35506982 Free PMC article. Review.
-
A versatile viral toolkit for functional discovery in the nervous system.Cell Rep Methods. 2022 May 26;2(6):100225. doi: 10.1016/j.crmeth.2022.100225. eCollection 2022 Jun 20. Cell Rep Methods. 2022. PMID: 35784651 Free PMC article.
-
Advances in CRISPR/Cas-based Gene Therapy in Human Genetic Diseases.Theranostics. 2020 Mar 15;10(10):4374-4382. doi: 10.7150/thno.43360. eCollection 2020. Theranostics. 2020. PMID: 32292501 Free PMC article. Review.
References
-
- Duan D, Yue Y, Engelhardt JF. Expanding AAV packaging capacity with trans-splicing or overlapping vectors: a quantitative comparison. Mol Ther. 2001;4(4):383–391. - PubMed
-
- Halbert CL, Allen JM, Miller AD. Efficient mouse airway transduction following recombination between AAV vectors carrying parts of a larger gene. Nat Biotechnol. 2002;20(7):697–701. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

