Longitudinal analysis of the peripheral B cell repertoire reveals unique effects of immunization with a new influenza virus strain
- PMID: 26608341
- PMCID: PMC4658769
- DOI: 10.1186/s13073-015-0239-y
Longitudinal analysis of the peripheral B cell repertoire reveals unique effects of immunization with a new influenza virus strain
Abstract
Background: Despite the potential to produce antibodies that can neutralize different virus (heterotypic neutralization), there is no knowledge of why vaccination against influenza induces protection predominantly against the utilized viral strains (homotypic response). Identification of structural patterns of the B cell repertoire associated to heterotypic neutralization may contribute to identify relevant epitopes for a universal vaccine against influenza.
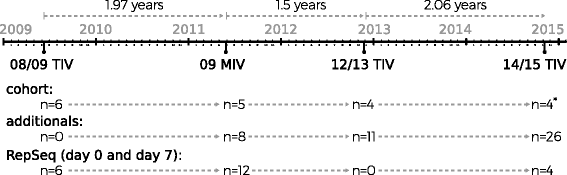
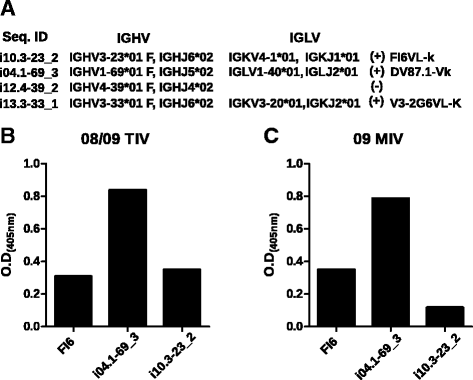
Methods: Blood samples were collected from volunteers immunized with 2008/2009 trivalent inactivated vaccine (TIV), pandemic H1N1 (pdmH1N1) monovalent inactivated vaccine (MIV) and the 2014/2015 TIV. Neutralization was assessed by hemagglutination and microneutralization test. IgG V(H) amplicons derived from peripheral blood RNA from pre-immune and 7 days post vaccination were subjected to 454-Roche sequencing. Full reconstruction of the sampled repertoires was done with ImmunediveRsity.
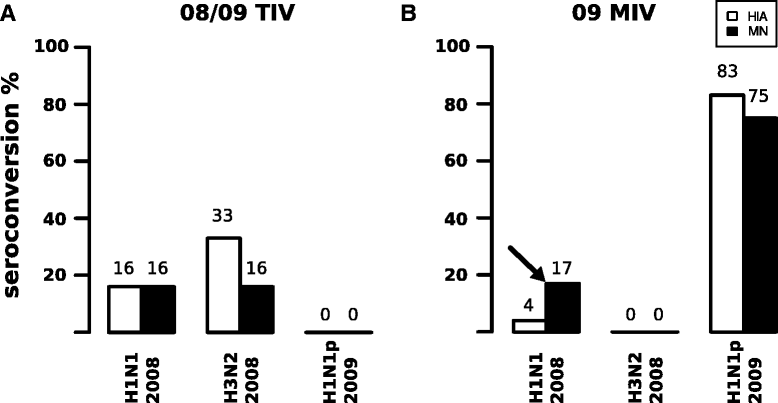
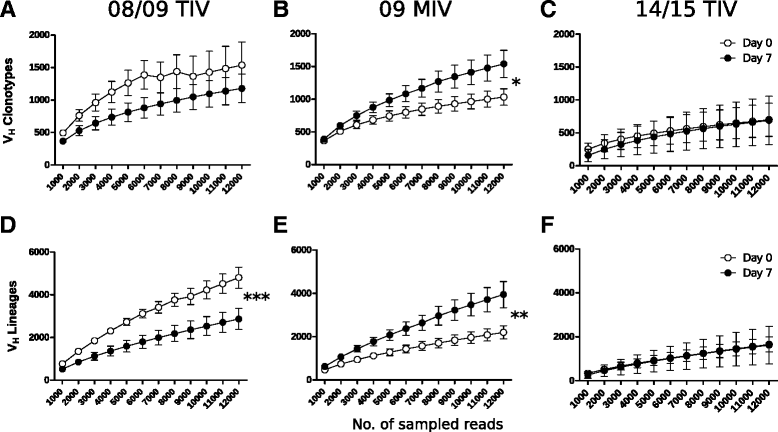
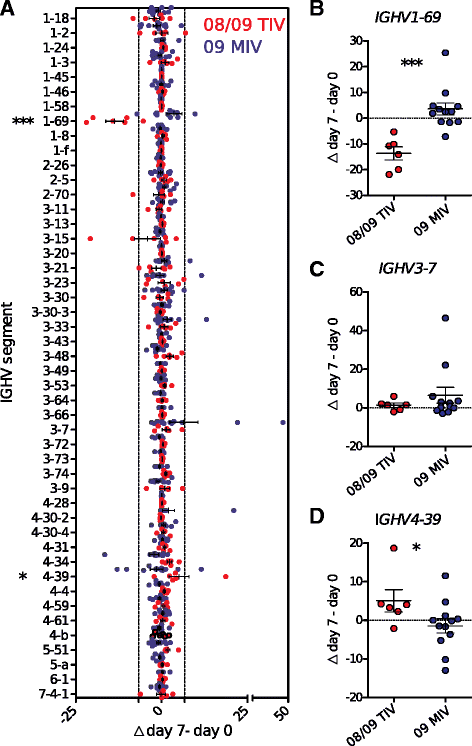
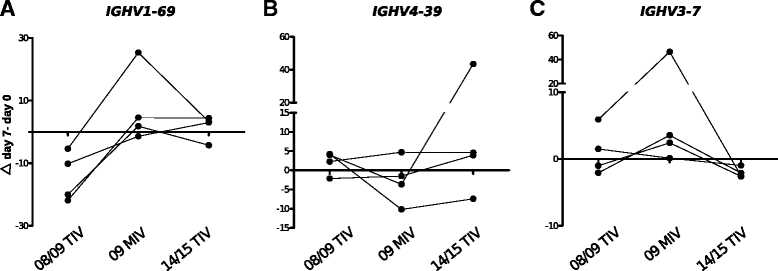
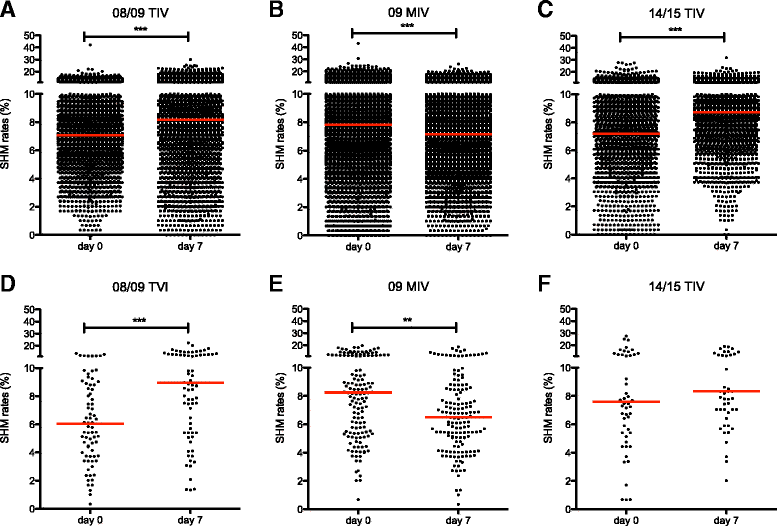
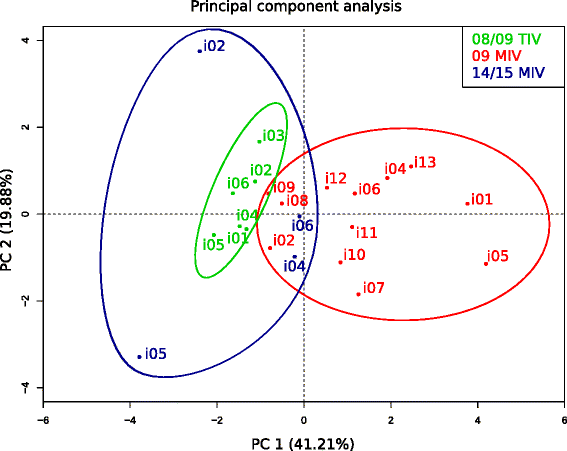
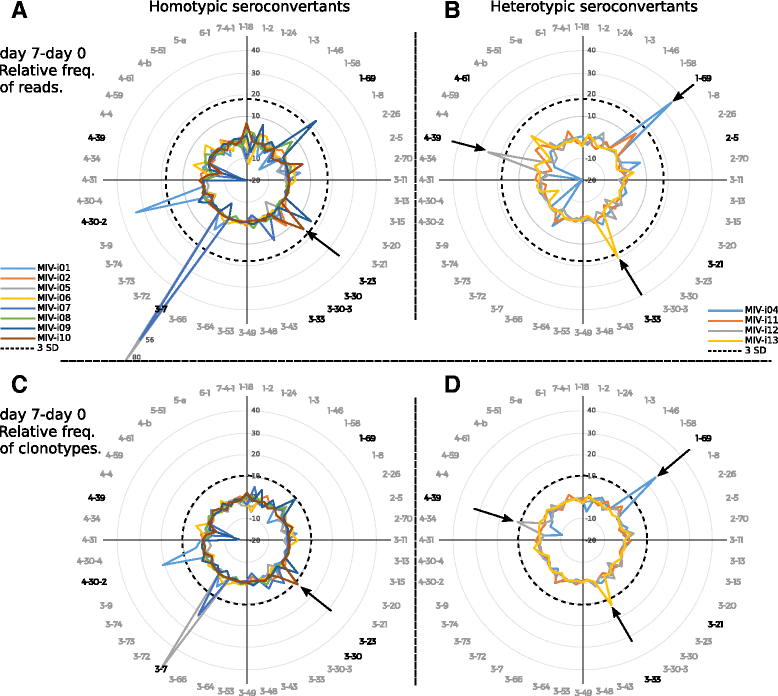
Results: The TIV induced a predominantly homotypic neutralizing serologic response, while the 09 MIV induced a heterotypic neutralizing seroconversion in 17% of the individuals. Both the 08/09 and the 14/15 TIV were associated with a reduction in clonotypic diversity, whereas 09 MIV was the opposite. Moreover, TIV and MIV induced distinctive patterns of IGHV segment use that are consistent with B cell selection by conserved antigenic determinants shared by the pre-pandemic and the pandemic strains. However, low somatic hypermutation rates in IgG after 09 MIV immunization, but not after 08/09 and 14/15 TIV immunization were observed. Furthermore, no evidence of the original antigenic sin was found in the same individuals after vaccination with the three vaccines.
Conclusions: Immunization with a new influenza virus strain (2009 pdmH1N1) induced unique effects in the peripheral B cell repertoire clonal structure, a stereotyped response involving distinctive IGHV segment use and low somatic hypermutation levels. These parameters were contrastingly different to those observed in response to pre-pandemic and post-pandemic vaccination, and may be the result of clonal selection of common antigenic determinants, as well as germinal center-independent responses that wane as the pandemic strain becomes seasonal. Our findings may contribute in the understanding of the structural and cellular basis required to develop a universal influenza vaccine.
Figures
Similar articles
-
Evaluation of T and B memory cell responses elicited by the pandemic H1N1 vaccine in HIV-infected and HIV-uninfected individuals.Vaccine. 2017 Oct 27;35(45):6103-6111. doi: 10.1016/j.vaccine.2017.09.059. Epub 2017 Oct 4. Vaccine. 2017. PMID: 28987439
-
Safety and immunogenicity of an AS03-adjuvanted A(H1N1)pmd09 vaccine administered simultaneously or sequentially with a seasonal trivalent vaccine in adults 61 years or older: data from two multicentre randomised trials.Vaccine. 2012 Oct 5;30(45):6483-91. doi: 10.1016/j.vaccine.2012.07.081. Epub 2012 Aug 9. Vaccine. 2012. PMID: 22885014 Clinical Trial.
-
Inactivated trivalent seasonal influenza vaccine induces limited cross-reactive neutralizing antibody responses against 2009 pandemic and 1934 PR8 H1N1 strains.Vaccine. 2010 Oct 4;28(42):6852-7. doi: 10.1016/j.vaccine.2010.08.031. Epub 2010 Aug 17. Vaccine. 2010. PMID: 20723626
-
Heads, stalks and everything else: how can antibodies eradicate influenza as a human disease?Curr Opin Immunol. 2016 Oct;42:48-55. doi: 10.1016/j.coi.2016.05.012. Epub 2016 Jun 3. Curr Opin Immunol. 2016. PMID: 27268395 Free PMC article. Review.
-
The road to a more effective influenza vaccine: Up to date studies and future prospects.Vaccine. 2017 Sep 25;35(40):5388-5395. doi: 10.1016/j.vaccine.2017.08.034. Epub 2017 Aug 31. Vaccine. 2017. PMID: 28866292 Review.
Cited by
-
Extraction of the CDRH3 sequence of the mouse antibody repertoire selected upon influenza virus infection by subtraction of the background antibody repertoire.J Virol. 2024 Mar 19;98(3):e0199523. doi: 10.1128/jvi.01995-23. Epub 2024 Feb 7. J Virol. 2024. PMID: 38323813 Free PMC article.
-
Immune imprinting and next-generation coronavirus vaccines.Nat Microbiol. 2023 Nov;8(11):1971-1985. doi: 10.1038/s41564-023-01505-9. Epub 2023 Nov 6. Nat Microbiol. 2023. PMID: 37932355 Review.
-
Characterizing the dynamics of BCR repertoire from repeated influenza vaccination.Emerg Microbes Infect. 2023 Dec;12(2):2245931. doi: 10.1080/22221751.2023.2245931. Emerg Microbes Infect. 2023. PMID: 37542407 Free PMC article.
-
Immune Imprinting and Implications for COVID-19.Vaccines (Basel). 2023 Apr 20;11(4):875. doi: 10.3390/vaccines11040875. Vaccines (Basel). 2023. PMID: 37112787 Free PMC article. Review.
-
A single residue in influenza virus H2 hemagglutinin enhances the breadth of the B cell response elicited by H2 vaccination.Nat Med. 2022 Feb;28(2):373-382. doi: 10.1038/s41591-021-01636-8. Epub 2022 Feb 3. Nat Med. 2022. PMID: 35115707
References
-
- WHO. Seasonal Flu: Fact sheet No. 211 OMS. Available at: http://www.who.int/mediacentre/factsheets/fs211/en/. Accessed 18 November 2015.
-
- CDC Estimates of deaths associated with Seasonal Influenza-United States, 1976-2007. Morb Mortal Wkly Rep. 2010;59(33):1057–62. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

