Directed Molecular Evolution of an Engineered Gammaretroviral Envelope Protein with Dual Receptor Use Shows Stable Maintenance of Both Receptor Specificities
- PMID: 26608314
- PMCID: PMC4719614
- DOI: 10.1128/JVI.02013-15
Directed Molecular Evolution of an Engineered Gammaretroviral Envelope Protein with Dual Receptor Use Shows Stable Maintenance of Both Receptor Specificities
Abstract
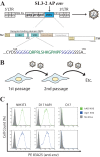
We have previously reported the construction of a murine leukemia virus-based replication-competent gammaretrovirus (SL3-AP) capable of utilizing the human G protein-coupled receptor APJ (hAPJ) as its entry receptor and its natural receptor, the murine Xpr1 receptor, with equal affinities. The apelin receptor has previously been shown to function as a coreceptor for HIV-1, and thus, adaptation of the viral vector to this receptor is of significant interest. Here, we report the molecular evolution of the SL3-AP envelope protein when the virus is cultured in cells harboring either the Xpr1 or the hAPJ receptor. Interestingly, the dual receptor affinity is maintained even after 10 passages in these cells. At the same time, the chimeric viral envelope protein evolves in a distinct pattern in the apelin cassette when passaged on D17 cells expressing hAPJ in three separate molecular evolution studies. This pattern reflects selection for reduced ligand-receptor interaction and is compatible with a model in which SL3-AP has evolved not to activate hAPJ receptor internalization.
Importance: Few successful examples of engineered retargeting of a retroviral vector exist. The engineered SL3-AP envelope is capable of utilizing either the murine Xpr1 or the human APJ receptor for entry. In addition, SL3-AP is the first example of an engineered retrovirus retaining its dual tropism after several rounds of passaging on cells expressing only one of its receptors. We demonstrate that the virus evolves toward reduced ligand-receptor affinity, which sheds new light on virus adaptation. We provide indirect evidence that such reduced affinity leads to reduced receptor internalization and propose a novel model in which too rapid receptor internalization may decrease virus entry.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures


Similar articles
-
Construction of a gammaretrovirus with a novel tropism and wild-type replication kinetics capable of using human APJ as entry receptor.J Virol. 2012 Oct;86(19):10621-7. doi: 10.1128/JVI.01028-12. Epub 2012 Jul 18. J Virol. 2012. PMID: 22811542 Free PMC article.
-
Coupling of receptor interference and a host-dependent post-binding entry deficiency in a gammaretroviral envelope protein.Retrovirology. 2010 Feb 5;7:9. doi: 10.1186/1742-4690-7-9. Retrovirology. 2010. PMID: 20137084 Free PMC article.
-
Wild mouse variants of envelope genes of xenotropic/polytropic mouse gammaretroviruses and their XPR1 receptors elucidate receptor determinants of virus entry.J Virol. 2007 Oct;81(19):10550-7. doi: 10.1128/JVI.00933-07. Epub 2007 Jul 18. J Virol. 2007. PMID: 17634227 Free PMC article.
-
Cell surface receptors for gammaretroviruses.Curr Top Microbiol Immunol. 2003;281:29-106. doi: 10.1007/978-3-642-19012-4_2. Curr Top Microbiol Immunol. 2003. PMID: 12932075 Review.
-
Library screening and receptor-directed targeting of gammaretroviral vectors.Future Microbiol. 2013 Jan;8(1):107-21. doi: 10.2217/fmb.12.122. Future Microbiol. 2013. PMID: 23252496 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

