Seasonality and selective trends in viral acute respiratory tract infections
- PMID: 26608252
- PMCID: PMC7116927
- DOI: 10.1016/j.mehy.2015.11.005
Seasonality and selective trends in viral acute respiratory tract infections
Abstract
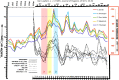
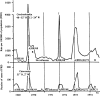
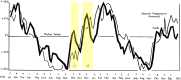
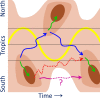
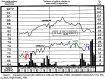
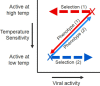
Influenza A and B, and many unrelated viruses including rhinovirus, RSV, adenovirus, metapneumovirus and coronavirus share the same seasonality, since these viral acute respiratory tract infections (vARIs) are much more common in winter than summer. Unfortunately, early investigations that used recycled "pedigree" virus strains seem to have led microbiologists to dismiss the common folk belief that vARIs often follow chilling. Today, incontrovertible evidence shows that ambient temperature dips and host chilling increase the incidence and severity of vARIs. This review considers four possible mechanisms, M1 - 4, that can explain this link: (M1) increased crowding in winter may enhance viral transmission; (M2) lower temperatures may increase the stability of virions outside the body; (M3) chilling may increase host susceptibility; (M4) lower temperatures or host chilling may activate dormant virions. There is little evidence for M1 or M2, which are incompatible with tropical observations. Epidemiological anomalies such as the repeated simultaneous arrival of vARIs over wide geographical areas, the rapid cessation of influenza epidemics, and the low attack rate of influenza within families are compatible with M4, but not M3 (in its simple form). M4 seems to be the main driver of seasonality, but M3 may also play an important role.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
Temperature dependent viral tropism: understanding viral seasonality and pathogenicity as applied to the avoidance and treatment of endemic viral respiratory illnesses.Rev Med Virol. 2022 Jan;32(1):e2241. doi: 10.1002/rmv.2241. Epub 2021 May 3. Rev Med Virol. 2022. PMID: 33942417 Free PMC article. Review.
-
[Etiological structure of influenza and other ARVI in St. Petersburg during epidemic seasons 2012-2016.].Vopr Virusol. 2018;63(5):233-239. doi: 10.18821/0507-4088-2018-63-5-233-239. Vopr Virusol. 2018. PMID: 30550100 Russian.
-
[Characteristics and the prevalence of respiratory viruses and the correlation with climatic factors of hospitalized children in Suzhou children's hospital].Zhonghua Yu Fang Yi Xue Za Zhi. 2011 Mar;45(3):205-10. Zhonghua Yu Fang Yi Xue Za Zhi. 2011. PMID: 21624230 Chinese.
-
Possible interference between seasonal epidemics of influenza and other respiratory viruses in Hong Kong, 2014-2017.BMC Infect Dis. 2017 Dec 16;17(1):772. doi: 10.1186/s12879-017-2888-5. BMC Infect Dis. 2017. PMID: 29246199 Free PMC article.
-
Correlations between climate factors and incidence--a contributor to RSV seasonality.Rev Med Virol. 2014 Jan;24(1):15-34. doi: 10.1002/rmv.1771. Epub 2013 Nov 9. Rev Med Virol. 2014. PMID: 24421259 Review.
Cited by
-
The role of vitamin D in reducing SARS-CoV-2 infection: An update.Int Immunopharmacol. 2021 Aug;97:107686. doi: 10.1016/j.intimp.2021.107686. Epub 2021 Apr 17. Int Immunopharmacol. 2021. PMID: 33930705 Free PMC article. Review.
-
Assessing the impact of air pollution and climate seasonality on COVID-19 multiwaves in Madrid, Spain.Environ Res. 2022 Jan;203:111849. doi: 10.1016/j.envres.2021.111849. Epub 2021 Aug 6. Environ Res. 2022. PMID: 34370990 Free PMC article.
-
Why is temperature sensitivity important for the success of common respiratory viruses?Rev Med Virol. 2021 Jan;31(1):1-8. doi: 10.1002/rmv.2153. Epub 2020 Aug 10. Rev Med Virol. 2021. PMID: 32776651 Free PMC article. Review.
-
Temperature dependent viral tropism: understanding viral seasonality and pathogenicity as applied to the avoidance and treatment of endemic viral respiratory illnesses.Rev Med Virol. 2022 Jan;32(1):e2241. doi: 10.1002/rmv.2241. Epub 2021 May 3. Rev Med Virol. 2022. PMID: 33942417 Free PMC article. Review.
-
Are Community Acquired Respiratory Viral Infections an Underestimated Burden in Hematology Patients?Microorganisms. 2019 Nov 2;7(11):521. doi: 10.3390/microorganisms7110521. Microorganisms. 2019. PMID: 31684063 Free PMC article. Review.
References
-
- Du Prel J.B., Puppe W., Gröndahl B., Knuf M., Weigl F., Schaaff F. Are meteorological parameters associated with acute respiratory tract infections? Clin Infect Dis. 2009;49(6):861–868. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

