Hypothalamic radial glia function as self-renewing neural progenitors in the absence of Wnt/β-catenin signaling
- PMID: 26603385
- PMCID: PMC4725207
- DOI: 10.1242/dev.126813
Hypothalamic radial glia function as self-renewing neural progenitors in the absence of Wnt/β-catenin signaling
Abstract
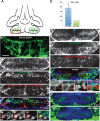
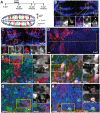
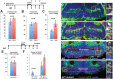
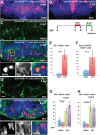
The vertebrate hypothalamus contains persistent radial glia that have been proposed to function as neural progenitors. In zebrafish, a high level of postembryonic hypothalamic neurogenesis has been observed, but the role of radial glia in generating these new neurons is unclear. We have used inducible Cre-mediated lineage labeling to show that a population of hypothalamic radial glia undergoes self-renewal and generates multiple neuronal subtypes at larval stages. Whereas Wnt/β-catenin signaling has been demonstrated to promote the expansion of other stem and progenitor cell populations, we find that Wnt/β-catenin pathway activity inhibits this process in hypothalamic radial glia and is not required for their self-renewal. By contrast, Wnt/β-catenin signaling is required for the differentiation of a specific subset of radial glial neuronal progeny residing along the ventricular surface. We also show that partial genetic ablation of hypothalamic radial glia or their progeny causes a net increase in their proliferation, which is also independent of Wnt/β-catenin signaling. Hypothalamic radial glia in the zebrafish larva thus exhibit several key characteristics of a neural stem cell population, and our data support the idea that Wnt pathway function may not be homogeneous in all stem or progenitor cells.
Keywords: Hypothalamus; Neural progenitors; Radial glia; Wnt signaling; Zebrafish.
© 2016. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures

Similar articles
-
Wnt/ß-catenin signaling is required for radial glial neurogenesis following spinal cord injury.Dev Biol. 2015 Jul 1;403(1):15-21. doi: 10.1016/j.ydbio.2015.03.025. Epub 2015 Apr 14. Dev Biol. 2015. PMID: 25888075 Free PMC article.
-
Wnt signaling regulates postembryonic hypothalamic progenitor differentiation.Dev Cell. 2012 Sep 11;23(3):624-36. doi: 10.1016/j.devcel.2012.07.012. Dev Cell. 2012. PMID: 22975330 Free PMC article.
-
Pax6 mediates ß-catenin signaling for self-renewal and neurogenesis by neocortical radial glial stem cells.Stem Cells. 2014 Jan;32(1):45-58. doi: 10.1002/stem.1561. Stem Cells. 2014. PMID: 24115331 Free PMC article.
-
Wnt/β-Catenin Signaling Pathway Governs a Full Program for Dopaminergic Neuron Survival, Neurorescue and Regeneration in the MPTP Mouse Model of Parkinson's Disease.Int J Mol Sci. 2018 Nov 24;19(12):3743. doi: 10.3390/ijms19123743. Int J Mol Sci. 2018. PMID: 30477246 Free PMC article. Review.
-
Mechanisms of radial glia progenitor cell lineage progression.FEBS Lett. 2017 Dec;591(24):3993-4008. doi: 10.1002/1873-3468.12906. Epub 2017 Nov 22. FEBS Lett. 2017. PMID: 29121403 Free PMC article. Review.
Cited by
-
Wnt/β-catenin signaling promotes neurogenesis in the diencephalospinal dopaminergic system of embryonic zebrafish.Sci Rep. 2022 Jan 19;12(1):1030. doi: 10.1038/s41598-022-04833-8. Sci Rep. 2022. PMID: 35046434 Free PMC article.
-
Midbrain tectal stem cells display diverse regenerative capacities in zebrafish.Sci Rep. 2019 Mar 14;9(1):4420. doi: 10.1038/s41598-019-40734-z. Sci Rep. 2019. PMID: 30872640 Free PMC article.
-
Dopaminergic neurons regenerate following chemogenetic ablation in the olfactory bulb of adult Zebrafish (Danio rerio).Sci Rep. 2020 Jul 30;10(1):12825. doi: 10.1038/s41598-020-69734-0. Sci Rep. 2020. PMID: 32733000 Free PMC article.
-
Stochastic cell-cycle entry and cell-state-dependent fate outputs of injury-reactivated tectal radial glia in zebrafish.Elife. 2019 Aug 23;8:e48660. doi: 10.7554/eLife.48660. Elife. 2019. PMID: 31442201 Free PMC article.
-
Motor Behavior Mediated by Continuously Generated Dopaminergic Neurons in the Zebrafish Hypothalamus Recovers after Cell Ablation.Curr Biol. 2016 Jan 25;26(2):263-269. doi: 10.1016/j.cub.2015.11.064. Epub 2016 Jan 7. Curr Biol. 2016. PMID: 26774784 Free PMC article.
References
-
- Agathocleous M., Iordanova I., Willardsen M. I., Xue X. Y., Vetter M. L., Harris W. A. and Moore K. B. (2009). A directional Wnt/beta-catenin-Sox2-proneural pathway regulates the transition from proliferation to differentiation in the Xenopus retina. Development 136, 3289-3299. 10.1242/dev.040451 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

