Oxysterol-binding Protein Activation at Endoplasmic Reticulum-Golgi Contact Sites Reorganizes Phosphatidylinositol 4-Phosphate Pools
- PMID: 26601944
- PMCID: PMC4714219
- DOI: 10.1074/jbc.M115.682997
Oxysterol-binding Protein Activation at Endoplasmic Reticulum-Golgi Contact Sites Reorganizes Phosphatidylinositol 4-Phosphate Pools
Abstract
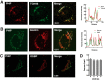
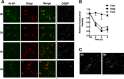
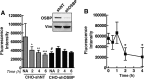
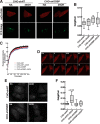
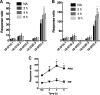
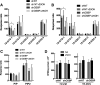
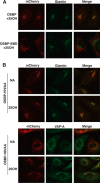
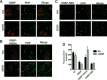
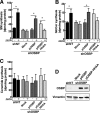
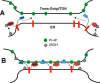
Oxysterol-binding protein (OSBP) exchanges cholesterol and phosphatidylinositol 4-phosphate (PI-4P) at contact sites between the endoplasmic reticulum (ER) and the trans-Golgi/trans-Golgi network. 25-Hydroxycholesterol (25OH) competitively inhibits this exchange reaction in vitro and causes the constitutive localization of OSBP at the ER/Golgi interface and PI-4P-dependent recruitment of ceramide transfer protein (CERT) for sphingomyelin synthesis. We used PI-4P probes and mass analysis to determine how OSBP controls the availability of PI-4P for this metabolic pathway. Treatment of fibroblasts or Chinese hamster ovary (CHO) cells with 25OH caused a 50-70% reduction in Golgi-associated immunoreactive PI-4P that correlated with Golgi localization of OSBP. In contrast, 25OH caused an OSBP-dependent enrichment in Golgi PI-4P that was detected with a pleckstrin homology domain probe. The cellular mass of phosphatidylinositol monophosphates and Golgi PI-4P measured with an unbiased PI-4P probe (P4M) was unaffected by 25OH and OSBP silencing, indicating that OSBP shifts the distribution of PI-4P upon localization to ER-Golgi contact sites. The PI-4P and sterol binding activities of OSBP were both required for 25OH activation of sphingomyelin synthesis, suggesting that 25OH must be exchanged for PI-4P to be concentrated at contact sites. We propose a model wherein 25OH activation of OSBP promotes the binding and retention of PI-4P at ER-Golgi contact sites. This pool of PI-4P specifically recruits pleckstrin homology domain-containing proteins involved in lipid transfer and metabolism, such as CERT.
Keywords: 25-hydroxycholesterol; Golgi; cholesterol; endoplasmic reticulum (ER); fluorescence recovery after photobleaching (FRAP); lipid transport; oxysterol binding protein; sphingomyelin.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

Similar articles
-
Oxysterol-binding protein recruitment and activity at the endoplasmic reticulum-Golgi interface are independent of Sac1.Traffic. 2017 Aug;18(8):519-529. doi: 10.1111/tra.12491. Epub 2017 May 31. Traffic. 2017. PMID: 28471037
-
Multisite phosphorylation of oxysterol-binding protein regulates sterol binding and activation of sphingomyelin synthesis.Mol Biol Cell. 2012 Sep;23(18):3624-35. doi: 10.1091/mbc.E12-04-0283. Epub 2012 Aug 8. Mol Biol Cell. 2012. PMID: 22875984 Free PMC article.
-
Oxysterol binding protein-dependent activation of sphingomyelin synthesis in the golgi apparatus requires phosphatidylinositol 4-kinase IIα.Mol Biol Cell. 2010 Dec;21(23):4141-50. doi: 10.1091/mbc.E10-05-0424. Epub 2010 Sep 29. Mol Biol Cell. 2010. PMID: 20881054 Free PMC article.
-
Phosphoinositides in the hepatitis C virus life cycle.Viruses. 2012 Oct 19;4(10):2340-58. doi: 10.3390/v4102340. Viruses. 2012. PMID: 23202467 Free PMC article. Review.
-
Structure, functions and regulation of CERT, a lipid-transfer protein for the delivery of ceramide at the ER-Golgi membrane contact sites.FEBS Lett. 2019 Sep;593(17):2366-2377. doi: 10.1002/1873-3468.13511. Epub 2019 Jul 8. FEBS Lett. 2019. PMID: 31254361 Review.
Cited by
-
SAC1 degrades its lipid substrate PtdIns4P in the endoplasmic reticulum to maintain a steep chemical gradient with donor membranes.Elife. 2018 Feb 20;7:e35588. doi: 10.7554/eLife.35588. Elife. 2018. PMID: 29461204 Free PMC article.
-
ORP1L mediated PI(4)P signaling at ER-lysosome-mitochondrion three-way contact contributes to mitochondrial division.Nat Commun. 2021 Sep 9;12(1):5354. doi: 10.1038/s41467-021-25621-4. Nat Commun. 2021. PMID: 34504082 Free PMC article.
-
Sterol transfer, PI4P consumption, and control of membrane lipid order by endogenous OSBP.EMBO J. 2017 Nov 2;36(21):3156-3174. doi: 10.15252/embj.201796687. Epub 2017 Oct 4. EMBO J. 2017. PMID: 28978670 Free PMC article.
-
Functions of Oxysterol-Binding Proteins at Membrane Contact Sites and Their Control by Phosphoinositide Metabolism.Front Cell Dev Biol. 2021 Jun 24;9:664788. doi: 10.3389/fcell.2021.664788. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34249917 Free PMC article. Review.
-
Ceramide Transfer Protein (CERT): An Overlooked Molecular Player in Cancer.Int J Mol Sci. 2021 Dec 7;22(24):13184. doi: 10.3390/ijms222413184. Int J Mol Sci. 2021. PMID: 34947980 Free PMC article. Review.
References
-
- Lagace T. A., and Ridgway N. D. (2013) The role of phospholipids in the biological activity and structure of the endoplasmic reticulum. Biochim. Biophys. Acta 1833, 2499–2510 - PubMed
-
- Holthuis J. C., and Menon A. K. (2014) Lipid landscapes and pipelines in membrane homeostasis. Nature 510, 48–57 - PubMed
-
- Olkkonen V. M., and Li S. (2013) Oxysterol-binding proteins: sterol and phosphoinositide sensors coordinating transport, signaling and metabolism. Prog. Lipid Res. 52, 529–538 - PubMed
-
- Alpy F., and Tomasetto C. (2014) START ships lipids across interorganelle space. Biochimie 96, 85–95 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

