Isolation of a novel bio-peptide from walnut residual protein inducing apoptosis and autophagy on cancer cells
- PMID: 26593407
- PMCID: PMC4656182
- DOI: 10.1186/s12906-015-0940-9
Isolation of a novel bio-peptide from walnut residual protein inducing apoptosis and autophagy on cancer cells
Abstract
Background: Walnut is unique because they have a perfect balance of n-6 and n-3 polyunsaturated fatty acids. The increasing market demand of walnut lipids results in the large amount of the oil extraction residue. The walnut residue is rich in nutritional proteins, and the uneconomic use of the by-product discouraged the development of walnut industry. Anticancer peptides have recently received attention as alternative chemotherapeutic agents that overcome the limits of current drugs. The aim of this study was to investigate whether anticancer bioactive peptide is contained in walnut.
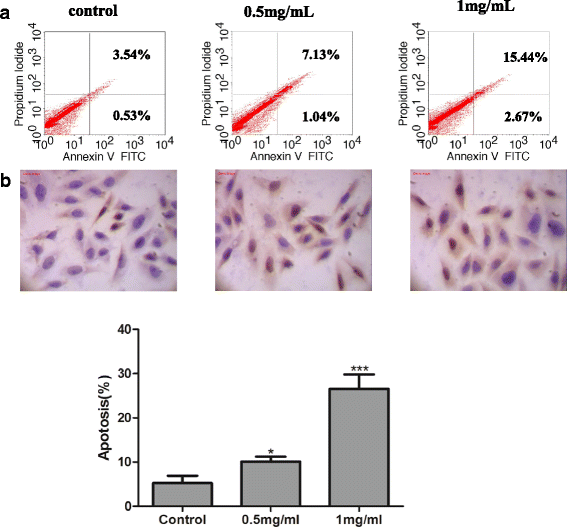
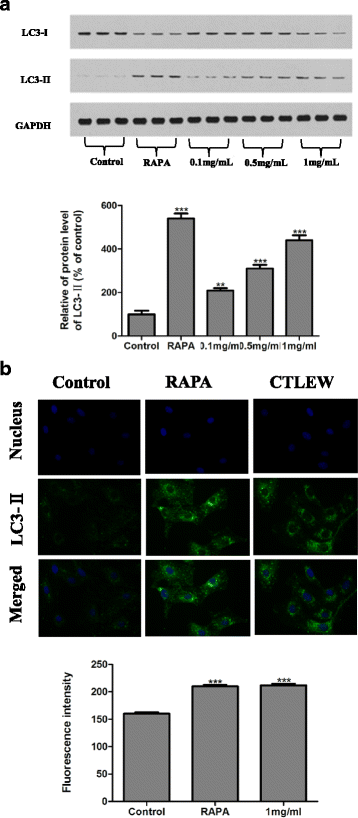
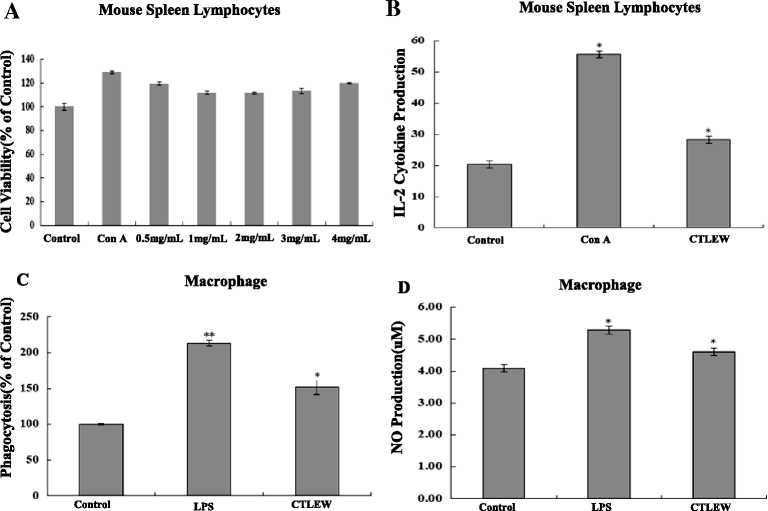
Methods: Walnut residual protein was hydrolyzed separately by five different proteases. The sequential purification of the hydrolysates was carried out by ultra-filtration, gel filtration chromatography and RP-HPLC to obtain a cancer cell growth inhibitory peptide. Cell cycle distribution, Annexin V-FITC/PI double staining, TUNEL assay, western blot and immunofluorescence for LC3-II assay were used to detect apoptosis and autophagy on cells. Cytokine production was measured by ELISA kits, macrophage phagocytosis was measured by neutral red uptake assay, nitric oxide production was measured by Griess reagent.
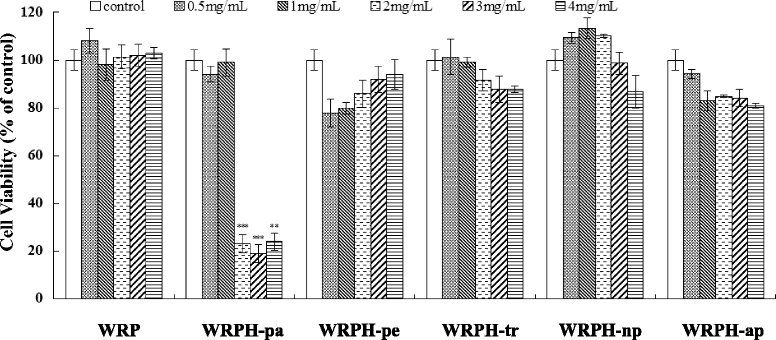
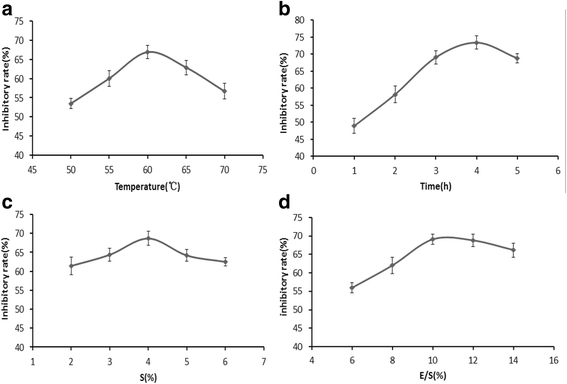
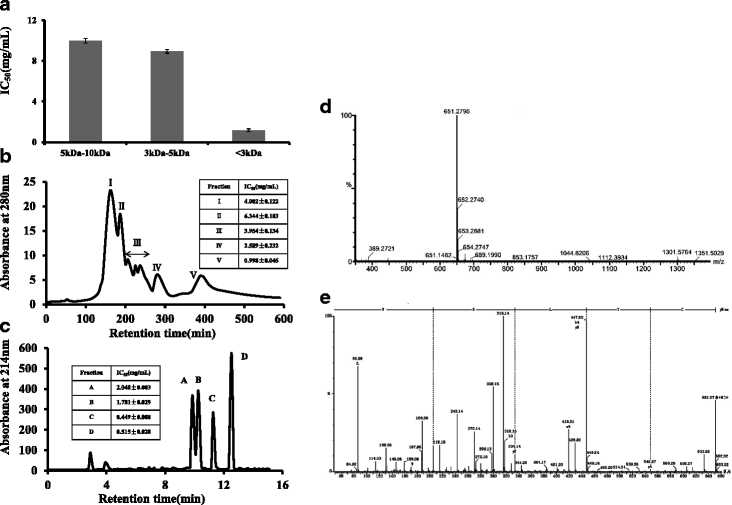
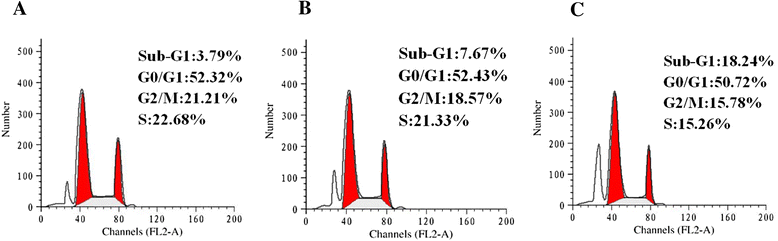
Results: The hydrolysates of walnut residual protein produced by papain under the optimal conditions (5 % substrate concentration and an enzyme-substrate ratio of 10 % at temperature 60 C for 3 h), showed significant growth inhibitory activity on MCF-7. The amino acid sequence of the purified peptide was identified as CTLEW with a molecular weight of 651.2795 Da. It is a novel bio-peptide with an amphiphilic structure. CTLEW induced both apoptosis and autophagy on MCF-7 cells, inhibited the cancer cells growth of Caco-2 and HeLa significantly, but did not show any cytotoxic activity against non-cancerous IEC-6 cells. Moreover, the bio-peptide enhanced proliferation and IL-2 secretion of spleen lymphocytes, promoted phagocytosis and NO production of macrophages.
Conclusion: These results suggested that a novel bio-peptide, CTLEW inducing apoptosis and autophagy on MCF-7 cells can be released from walnut residual protein through papain hydrolyzing under the certain condition. The bio-peptide shows selective inhibition towards cancer cells growth and immunomodulatory activity.
Figures
Similar articles
-
Purification and identification of antioxidant peptides from walnut (Juglans regia L.) protein hydrolysates.Peptides. 2012 Dec;38(2):344-9. doi: 10.1016/j.peptides.2012.09.017. Epub 2012 Sep 26. Peptides. 2012. PMID: 23022588
-
Antioxidant and Anticancer Activities of Walnut (Juglans regia L.) Protein Hydrolysates Using Different Proteases.Plant Foods Hum Nutr. 2016 Dec;71(4):402-409. doi: 10.1007/s11130-016-0576-z. Plant Foods Hum Nutr. 2016. PMID: 27679440 Free PMC article.
-
Purification and identification of an ACE inhibitory peptide from walnut protein.J Agric Food Chem. 2013 May 1;61(17):4097-100. doi: 10.1021/jf4001378. Epub 2013 Apr 19. J Agric Food Chem. 2013. PMID: 23566262
-
Bioactive Peptides from Walnut Residue Protein.Molecules. 2020 Mar 12;25(6):1285. doi: 10.3390/molecules25061285. Molecules. 2020. PMID: 32178315 Free PMC article. Review.
-
Walnut Protein: A Rising Source of High-Quality Protein and Its Updated Comprehensive Review.J Agric Food Chem. 2023 Jul 19;71(28):10525-10542. doi: 10.1021/acs.jafc.3c01620. Epub 2023 Jul 3. J Agric Food Chem. 2023. PMID: 37399339 Review.
Cited by
-
The Updated Review on Plant Peptides and Their Applications in Human Health.Int J Pept Res Ther. 2022;28(5):135. doi: 10.1007/s10989-022-10437-7. Epub 2022 Jul 27. Int J Pept Res Ther. 2022. PMID: 35911180 Free PMC article. Review.
-
The Gastroprotective Effect of Small Molecule Oligopeptides Isolated from Walnut (Juglans regia L.) against Ethanol-Induced Gastric Mucosal Injury in Rats.Nutrients. 2020 Apr 18;12(4):1138. doi: 10.3390/nu12041138. Nutrients. 2020. PMID: 32325708 Free PMC article.
-
Antioxidant Function and Application of Plant-Derived Peptides.Antioxidants (Basel). 2024 Oct 6;13(10):1203. doi: 10.3390/antiox13101203. Antioxidants (Basel). 2024. PMID: 39456457 Free PMC article. Review.
-
A Concise Review on the Role of Natural and Synthetically Derived Peptides in Colorectal Cancer.Curr Top Med Chem. 2022;22(31):2571-2588. doi: 10.2174/1568026622666220516105049. Curr Top Med Chem. 2022. PMID: 35578849 Review.
-
Integrated Transcriptomic and Proteomic Analysis of Nutritional Quality-Related Molecular Mechanisms in "Longjia", "Yangpao", and "Niangqing" Walnuts (Juglans sigillata).Int J Mol Sci. 2024 Oct 30;25(21):11671. doi: 10.3390/ijms252111671. Int J Mol Sci. 2024. PMID: 39519221 Free PMC article.
References
-
- Martínez ML, Labuckas DO, Lamarque AL, Maestri DM. Walnut (Juglans regia L.): Genetic resources, chemistry, by-products. J Sci Food Agric. 2010;90(12):1959–1967. - PubMed
-
- Gu M, Chen H, Zhao M, Wang X, Yang B, Ren J, et al. Identification of antioxidant peptides released from defatted walnut (Juglans Sigillata Dode) meal proteins with pancreatin. LWT-Food Sci Technol. 2015;60(1):213–20. doi: 10.1016/j.lwt.2014.07.052. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

