Designing a broad-spectrum integrative approach for cancer prevention and treatment
- PMID: 26590477
- PMCID: PMC4819002
- DOI: 10.1016/j.semcancer.2015.09.007
Designing a broad-spectrum integrative approach for cancer prevention and treatment
Abstract
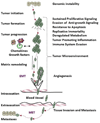
Targeted therapies and the consequent adoption of "personalized" oncology have achieved notable successes in some cancers; however, significant problems remain with this approach. Many targeted therapies are highly toxic, costs are extremely high, and most patients experience relapse after a few disease-free months. Relapses arise from genetic heterogeneity in tumors, which harbor therapy-resistant immortalized cells that have adopted alternate and compensatory pathways (i.e., pathways that are not reliant upon the same mechanisms as those which have been targeted). To address these limitations, an international task force of 180 scientists was assembled to explore the concept of a low-toxicity "broad-spectrum" therapeutic approach that could simultaneously target many key pathways and mechanisms. Using cancer hallmark phenotypes and the tumor microenvironment to account for the various aspects of relevant cancer biology, interdisciplinary teams reviewed each hallmark area and nominated a wide range of high-priority targets (74 in total) that could be modified to improve patient outcomes. For these targets, corresponding low-toxicity therapeutic approaches were then suggested, many of which were phytochemicals. Proposed actions on each target and all of the approaches were further reviewed for known effects on other hallmark areas and the tumor microenvironment. Potential contrary or procarcinogenic effects were found for 3.9% of the relationships between targets and hallmarks, and mixed evidence of complementary and contrary relationships was found for 7.1%. Approximately 67% of the relationships revealed potentially complementary effects, and the remainder had no known relationship. Among the approaches, 1.1% had contrary, 2.8% had mixed and 62.1% had complementary relationships. These results suggest that a broad-spectrum approach should be feasible from a safety standpoint. This novel approach has potential to be relatively inexpensive, it should help us address stages and types of cancer that lack conventional treatment, and it may reduce relapse risks. A proposed agenda for future research is offered.
Keywords: Cancer hallmarks; Integrative medicine; Multi-targeted; Phytochemicals; Targeted therapy.
Copyright © 2015 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Keith Block is an owner of the Block Center for Integrative Cancer Treatment and of North Shore Nutraceuticals; Charlotte Gyllenhaal is an employee of the Block Center for Integrative Cancer Treatment; Jack Arbiser is the inventor of US Patents involving derivatives of honokiol and NADPH oxidase inhibitors. He has also cofounded ABBY Therapeutics for the development of NADPH oxidase inhibitors; Penny Block is the Executive Director of the Block Center for Integrative Cancer Treatment and President of North Shore Nutraceuticals; Ralph J. DeBerardinis is a member of the scientific advisory boards for Peloton Therapeutics and Agios Pharmaceuticals; Anna Mae E. Diehl has grants from Shire-Research, Metabolon, and Gilead. She is also a consultant for Astrazeneca, Genentech, Japan Tobacco, and the NuSI Foundation; Byoung S. Kwon holds patents for methods regarding anti-CD 137 and adaptive CTL therapeutics; Valter D. Longo has an equity interest in L-Nutra, a company that develops medical food; Kapil Mehta is a scientific advisor to Lifecare Innovations, and holds India Patent 8.765.797, TG2 inhibitors and uses thereof; Michael P. Murphy holds intellectual property in mitochondrial therapies and has ownership shares in a company called Antipodean Pharmaceuticals Inc. which is trying to commercialize some of these compounds; Jeffrey C. Rathmell received indirect compensation from Novartis while working on this project; Luigi Ricciardiello received an unrestricted research grant from SLA Pharma AG, Switzerland.; John Stagg has a sponsored research agreement with Medimmune LLC and is on the scientific advisory board of Surface Oncology; Matthew G. Vander Heiden is a consultant, scientific advisory board member, and owns equity in Agios Pharmaceuticals
Figures
Similar articles
-
A broad-spectrum integrative design for cancer prevention and therapy: The challenge ahead.Semin Cancer Biol. 2015 Dec;35 Suppl:S1-S4. doi: 10.1016/j.semcancer.2015.08.002. Epub 2015 Aug 7. Semin Cancer Biol. 2015. PMID: 26260004
-
A multi-targeted approach to suppress tumor-promoting inflammation.Semin Cancer Biol. 2015 Dec;35 Suppl:S151-S184. doi: 10.1016/j.semcancer.2015.03.006. Epub 2015 May 5. Semin Cancer Biol. 2015. PMID: 25951989 Free PMC article. Review.
-
Broad targeting of angiogenesis for cancer prevention and therapy.Semin Cancer Biol. 2015 Dec;35 Suppl(Suppl):S224-S243. doi: 10.1016/j.semcancer.2015.01.001. Epub 2015 Jan 16. Semin Cancer Biol. 2015. PMID: 25600295 Free PMC article. Review.
-
Integrative therapies in cancer: modulating a broad spectrum of targets for cancer management.Integr Cancer Ther. 2015 Mar;14(2):113-8. doi: 10.1177/1534735414567473. Epub 2015 Jan 18. Integr Cancer Ther. 2015. PMID: 25601968
-
Addressing intra-tumoral heterogeneity and therapy resistance.Oncotarget. 2016 Nov 1;7(44):72322-72342. doi: 10.18632/oncotarget.11875. Oncotarget. 2016. PMID: 27608848 Free PMC article. Review.
Cited by
-
A systems approach to personalised nutrition: Report on the Keystone Symposium "Human Nutrition, Environment and Health".Appl Transl Genom. 2016 Aug 4;10:16-8. doi: 10.1016/j.atg.2016.08.001. eCollection 2016 Sep. Appl Transl Genom. 2016. PMID: 27668171 Free PMC article. No abstract available.
-
Novel application of metformin combined with targeted drugs on anticancer treatment.Cancer Sci. 2019 Jan;110(1):23-30. doi: 10.1111/cas.13849. Epub 2018 Nov 28. Cancer Sci. 2019. PMID: 30358009 Free PMC article. Review.
-
Targeting the Warburg effect for cancer treatment: Ketogenic diets for management of glioma.Semin Cancer Biol. 2019 Jun;56:135-148. doi: 10.1016/j.semcancer.2017.12.011. Epub 2017 Dec 30. Semin Cancer Biol. 2019. PMID: 29294371 Free PMC article. Review.
-
Flaxseed Lignans as Important Dietary Polyphenols for Cancer Prevention and Treatment: Chemistry, Pharmacokinetics, and Molecular Targets.Pharmaceuticals (Basel). 2019 May 5;12(2):68. doi: 10.3390/ph12020068. Pharmaceuticals (Basel). 2019. PMID: 31060335 Free PMC article. Review.
-
Combination of Polymeric Micelle Formulation of TGFβ Receptor Inhibitors and Paclitaxel Produce Consistent Response Across Different Mouse Models of TNBC.bioRxiv [Preprint]. 2023 Jun 14:2023.06.14.544381. doi: 10.1101/2023.06.14.544381. bioRxiv. 2023. Update in: Bioeng Transl Med. 2024 Jun 04;9(5):e10681. doi: 10.1002/btm2.10681. PMID: 37398150 Free PMC article. Updated. Preprint.
References
-
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. Lyon, France: International Agency for Research on Cancer; [cited 17 July 2014]. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet] Available from http://globocan.iarc.fr.
-
- Block KI. Life Over Cancer. New York: Bantam; 2009. p. 594. 2009.
-
- Kruse V, Rottey S, De Backer O, Van Belle S, Cocquyt V, Denys H. PARP inhibitors in oncology: a new synthetic lethal approach to cancer therapy. Acta Clin Belg. 2011;66(1):2–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01AG034906/AG/NIA NIH HHS/United States
- R01 CA164095/CA/NCI NIH HHS/United States
- P01 AG034906/AG/NIA NIH HHS/United States
- R01GM071725/GM/NIGMS NIH HHS/United States
- R01CA100816/CA/NCI NIH HHS/United States
- G1200MD007581/MD/NIMHD NIH HHS/United States
- U01 ES019458/ES/NIEHS NIH HHS/United States
- R01 AI076535/AI/NIAID NIH HHS/United States
- K01AT007324/AT/NCCIH NIH HHS/United States
- 1R01CA151304/CA/NCI NIH HHS/United States
- R01 CA131294/CA/NCI NIH HHS/United States
- P30AG028716-01/AG/NIA NIH HHS/United States
- R01-HL107652/HL/NHLBI NIH HHS/United States
- R01AG020642/AG/NIA NIH HHS/United States
- T32HL098062/HL/NHLBI NIH HHS/United States
- K01 AT007324/AT/NCCIH NIH HHS/United States
- F31 CA154080/CA/NCI NIH HHS/United States
- R03 CA171326/CA/NCI NIH HHS/United States
- ES019458/ES/NIEHS NIH HHS/United States
- R01 HL108006/HL/NHLBI NIH HHS/United States
- T32 CA059365/CA/NCI NIH HHS/United States
- T32 HL098062/HL/NHLBI NIH HHS/United States
- T32-CA059365-19/CA/NCI NIH HHS/United States
- R01 CA100816/CA/NCI NIH HHS/United States
- 1P01AT003961/AT/NCCIH NIH HHS/United States
- R01HL108006/HL/NHLBI NIH HHS/United States
- U54 CA143907/CA/NCI NIH HHS/United States
- R21 CA155686/CA/NCI NIH HHS/United States
- R01 CA170378/CA/NCI NIH HHS/United States
- R33 CA161873-02/CA/NCI NIH HHS/United States
- T32 HL134632/HL/NHLBI NIH HHS/United States
- R21CA169757/CA/NCI NIH HHS/United States
- R21CA184788/CA/NCI NIH HHS/United States
- R15 CA137499/CA/NCI NIH HHS/United States
- CA164095/CA/NCI NIH HHS/United States
- R21 CA169757/CA/NCI NIH HHS/United States
- R03DK089130/DK/NIDDK NIH HHS/United States
- P20RR016477/RR/NCRR NIH HHS/United States
- 5R01CA127258-05/CA/NCI NIH HHS/United States
- R33 CA161873/CA/NCI NIH HHS/United States
- R01 CA028704/CA/NCI NIH HHS/United States
- R01CA170378/CA/NCI NIH HHS/United States
- R01CA166348/CA/NCI NIH HHS/United States
- 5P01CA073992/CA/NCI NIH HHS/United States
- R01 CA166348/CA/NCI NIH HHS/United States
- MC_UU_12022/6/MRC_/Medical Research Council/United Kingdom
- MOP114962/CAPMC/ CIHR/Canada
- K08 NS083732/NS/NINDS NIH HHS/United States
- CA-10951/CA/NCI NIH HHS/United States
- R01 CA156776/CA/NCI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- MC_U105663142/MRC_/Medical Research Council/United Kingdom
- R00 CA168997/CA/NCI NIH HHS/United States
- R01 AG020642/AG/NIA NIH HHS/United States
- CA168997/CA/NCI NIH HHS/United States
- U54CA149145/CA/NCI NIH HHS/United States
- R03 DK089130/DK/NIDDK NIH HHS/United States
- F32 CA177139/CA/NCI NIH HHS/United States
- MOP125857/CAPMC/ CIHR/Canada
- 1R01CA20009/CA/NCI NIH HHS/United States
- R01 AG045351/AG/NIA NIH HHS/United States
- K01DK077137/DK/NIDDK NIH HHS/United States
- P01 AT003961/AT/NCCIH NIH HHS/United States
- MC_UP_1101/3/MRC_/Medical Research Council/United Kingdom
- R01CA156776/CA/NCI NIH HHS/United States
- R01 AI110613/AI/NIAID NIH HHS/United States
- P20 RR016477/RR/NCRR NIH HHS/United States
- K01 DK077137/DK/NIDDK NIH HHS/United States
- P01AG034906-01A1/AG/NIA NIH HHS/United States
- MSH-136647/CAPMC/ CIHR/Canada
- P30 CA022453/CA/NCI NIH HHS/United States
- ImNIH/Intramural NIH HHS/United States
- R01 CA109335-04A1/CA/NCI NIH HHS/United States
- P20GM103434/GM/NIGMS NIH HHS/United States
- R21 CA184788/CA/NCI NIH HHS/United States
- R01 HL107652/HL/NHLBI NIH HHS/United States
- F32CA177139/CA/NCI NIH HHS/United States
- P01 CA073992/CA/NCI NIH HHS/United States
- R01CA131294/CA/NCI NIH HHS/United States
- P30 AG028716/AG/NIA NIH HHS/United States
- U54 CA149145/CA/NCI NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- R01 CA109335/CA/NCI NIH HHS/United States
- R01 CA127258/CA/NCI NIH HHS/United States
- P20 GM103434/GM/NIGMS NIH HHS/United States
- F31CA154080/CA/NCI NIH HHS/United States
- G12 MD007581/MD/NIMHD NIH HHS/United States
- R01 GM071725/GM/NIGMS NIH HHS/United States
- R21 CA188818/CA/NCI NIH HHS/United States
- MOP 64308/CAPMC/ CIHR/Canada
- AI076535/AI/NIAID NIH HHS/United States
- R01 AR047901/AR/NIAMS NIH HHS/United States
- C301/A14762/CRUK_/Cancer Research UK/United Kingdom
- R01 CA120009/CA/NCI NIH HHS/United States
- R01 CA151304/CA/NCI NIH HHS/United States
- U54CA143907/CA/NCI NIH HHS/United States
- 1R03CA1711326/CA/NCI NIH HHS/United States
- 5T32-CA059365/CA/NCI NIH HHS/United States
- R21CA188818/CA/NCI NIH HHS/United States
- I01 BX001517/BX/BLRD VA/United States
- P30 CA22453/CA/NCI NIH HHS/United States
- AR47901/AR/NIAMS NIH HHS/United States
- K08NS083732/NS/NINDS NIH HHS/United States
- R15 CA137499-01/CA/NCI NIH HHS/United States
- R01 CA010951/CA/NCI NIH HHS/United States

