Pathology of Equine Influenza virus (H3N8) in Murine Model
- PMID: 26587990
- PMCID: PMC4654517
- DOI: 10.1371/journal.pone.0143094
Pathology of Equine Influenza virus (H3N8) in Murine Model
Abstract
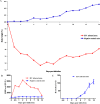
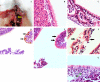
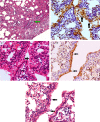
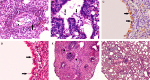
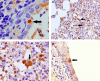
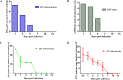
Equine influenza viruses (EIV)-H3N8 continue to circulate in equine population throughout the world. They evolve by the process of antigenic drift that leads to substantial change in the antigenicity of the virus, thereby necessitating substitution of virus strain in the vaccines. This requires frequent testing of the new vaccines in the in vivo system; however, lack of an appropriate laboratory animal challenge model for testing protective efficacy of equine influenza vaccine candidates hinders the screening of new vaccines and other therapeutic approaches. In the present investigation, BALB/c mouse were explored for suitability for conducting pathogenecity studies for EIV. The BALB/c mice were inoculated intranasally @ 2×106.24 EID50 with EIV (H3N8) belonging to Clade 2 of Florida sublineage and monitored for setting up of infection and associated parameters. All mice inoculated with EIV exhibited clinical signs viz. loss in body weights, lethargy, dyspnea, etc, between 3 and 5 days which commensurate with lesions observed in the respiratory tract including rhinitis, tracheitis, bronchitis, bronchiolitis, alveolitis and diffuse interstitial pneumonia. Transmission electron microscopy, immunohistochemistry, virus quantification through titration and qRT-PCR demonstrated active viral infection in the upper and lower respiratory tract. Serology revealed rise in serum lactate dehydrogenase levels along with sero-conversion. The pattern of disease progression, pathological lesions and virus recovery from nasal washings and lungs in the present investigations in mice were comparable to natural and experimental EIV infection in equines. The findings establish BALB/c mice as small animal model for studying EIV (H3N8) infection and will have immense potential for dissecting viral pathogenesis, vaccine efficacy studies, preliminary screening of vaccine candidates and antiviral therapeutics against EIV.
Conflict of interest statement
Figures


Similar articles
-
Immunogenicity and protective efficacy of inactivated equine influenza (H3N8) virus vaccine in murine model.Vet Microbiol. 2017 Oct;210:188-196. doi: 10.1016/j.vetmic.2017.08.013. Epub 2017 Aug 26. Vet Microbiol. 2017. PMID: 29103691
-
Pathological and immunological protection induced by inactivated reverse genetics-based H3N8 equine influenza vaccine candidate in murine model.Acta Virol. 2020;64(3):359-374. doi: 10.4149/av_2020_314. Acta Virol. 2020. PMID: 32985215
-
Refinement of the equine influenza model in the natural host: A meta-analysis to determine the benefits of individual nebulisation for experimental infection and vaccine evaluation in the face of decreased strain pathogenicity.Vet Microbiol. 2017 Nov;211:150-159. doi: 10.1016/j.vetmic.2017.10.010. Epub 2017 Oct 10. Vet Microbiol. 2017. PMID: 29102112
-
A Brief Introduction to Equine Influenza and Equine Influenza Viruses.Methods Mol Biol. 2020;2123:355-360. doi: 10.1007/978-1-0716-0346-8_26. Methods Mol Biol. 2020. PMID: 32170701 Review.
-
Equine Influenza Virus and Vaccines.Viruses. 2021 Aug 20;13(8):1657. doi: 10.3390/v13081657. Viruses. 2021. PMID: 34452521 Free PMC article. Review.
Cited by
-
Equine Influenza.Cold Spring Harb Perspect Med. 2022 Jan 4;12(1):a038331. doi: 10.1101/cshperspect.a038331. Cold Spring Harb Perspect Med. 2022. PMID: 32152243 Free PMC article. Review.
-
Effect of the selection pressure of vaccine antibodies on evolution of H9N2 avian influenza virus in chickens.AMB Express. 2020 May 27;10(1):98. doi: 10.1186/s13568-020-01036-0. AMB Express. 2020. PMID: 32462233 Free PMC article.
-
Protective efficacy of inactivated reverse genetics based equine influenza vaccine candidate adjuvanted with MontanideTM Pet Gel in murine model.J Vet Med Sci. 2019 Dec 18;81(12):1753-1762. doi: 10.1292/jvms.19-0399. Epub 2019 Oct 28. J Vet Med Sci. 2019. PMID: 31656240 Free PMC article.
-
Evaluation of concurrent vaccinations with recombinant canarypox equine influenza virus and inactivated equine herpesvirus vaccines.J Anim Sci Technol. 2022 May;64(3):588-598. doi: 10.5187/jast.2022.e30. Epub 2022 May 31. J Anim Sci Technol. 2022. PMID: 35709134 Free PMC article.
-
A Quadruple Gene-Deleted Live BoHV-1 Subunit RVFV Vaccine Vector Reactivates from Latency and Replicates in the TG Neurons of Calves but Is Not Transported to and Shed from Nasal Mucosa.Viruses. 2024 Sep 21;16(9):1497. doi: 10.3390/v16091497. Viruses. 2024. PMID: 39339973 Free PMC article.
References
-
- Mumford JA, Chambers TM. Equine Influenza In: Textbook of influenza. Blackwell Healthcare Communication;1998. p. 146–62.
-
- Gerber H. Clinical features sequelae and epidemiology of equine influenza In: Bryans JT, Gerber H, editors. Equine Infectious Diseases II. Switzerland: S. Karger; 1970. p. 63–80.
-
- Webster RG. Are equine 1 influenza viruses still present in horses? Equine Vet J. 1993;25: 537–8. - PubMed
-
- OIE Terrestrial Manual 2012, Chapter 2.5.7. Equine influenza.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

