HIF-1α pathway: role, regulation and intervention for cancer therapy
- PMID: 26579469
- PMCID: PMC4629436
- DOI: 10.1016/j.apsb.2015.05.007
HIF-1α pathway: role, regulation and intervention for cancer therapy
Abstract
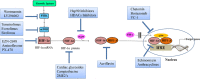
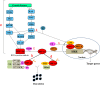
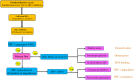
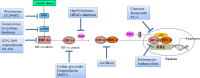
Hypoxia-inducible factor-1 (HIF-1) has been recognized as an important cancer drug target. Many recent studies have provided convincing evidences of strong correlation between elevated levels of HIF-1 and tumor metastasis, angiogenesis, poor patient prognosis as well as tumor resistance therapy. It was found that hypoxia (low O2 levels) is a common character in many types of solid tumors. As an adaptive response to hypoxic stress, hypoxic tumor cells activate several survival pathways to carry out their essential biological processes in different ways compared with normal cells. Recent advances in cancer biology at the cellular and molecular levels highlighted the HIF-1α pathway as a crucial survival pathway for which novel strategies of cancer therapy could be developed. However, targeting the HIF-1α pathway has been a challenging but promising progresses have been made in the past twenty years. This review summarizes the role and regulation of the HIF-1α in cancer, and recent therapeutic approaches targeting this important pathway.
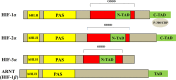
Keywords: 4E-BP1, eukaryotic translation initiation factor 4E (eIF-4E) binding protein p70 S6 kinase (S6K); ADM, adrenomedullin; AKt, protein kinase B; ARD-1, arrest-defective-1; ARNT, aryl hydrocarbon nuclear translocator; AhR, aryl hydrocarbon receptor; C-MYC, myelocytomatosis virus oncogene cellular homolog; C-TAD, COOH-terminal TAD; CAC, circulating angiogenic cells; CPTs, camptothecins; Cancer drug discovery and development; ChIP, chromatin immunoprecipitation; CoCl2, cobalt chloride; DFO, deferoxamine; EGF, epidermal growth factor; ELISA, enzyme-linked immunosorbent assay; EMSA, electrophoretic mobility shift assay; EPO, erythropoietin; ERK, extracellular signal-regulated kinase; FIH-1, factor inhibiting HIF-1; GA, geldanamycin; GAs, geldanamycins; GLUT1, glucose transporter 1; GLUT3, glucose transporter 3; GLUTs, glucose transporters; HDAC, histone deacetylase; HIF-1α; HIF-1α inhibitors; HIF-1α, hypoxia-inducible factor-1α; HK1, hexokinase 1; HK2, hexokinase 2; HPH, HIF-1 prolyl hydroxylases; HRE, hypoxia response elements; HTS, high throughput screens; Hsp90, heat shock protein 90; ID2, DNA-binding protein inhibitor; IGF-BP2, IGF-factor-binding protein 2; IGF-BP3, IGF-factor-binding protein 3; IGF2, insulin-like growth factor 2; IPAS, inhibitory PAS; K, lysine residue; LDHA, lactate dehydrogenase; LEP, leptin; LRP1, LDL-receptor-related protein 1; Luc, luciferase; MAPK, mitogen-activated protein kinases; MEK, MAPK/ERK kinase; MNK, MAP kinase interacting kinase; MTs, microtubules; Mdm2, mouse double minute 2 homolog; N, asparagine residue; N-TAD, NH2-terminal TAD; NOS, nitric oxide synthase; ODDD, oxygen dependent degradation domain; P, proline residue; PAS, Per and Sim; PCAF, p300/CBP associated factor; PHDs, prolyl-4-hydroxylases; PI3K, phosphatidyl inositol-4,5-bisphosphate-3-kinase; PKM, pyruvate kinase M; RCC, renal cell carcinoma; RT-PCR, reverse transcription polymerase chain reaction; Raf, rapidly accelerated fibrosarcoma; Ras, rat sarcoma; SIRT 1, Sirtuin 1; TAD, transactivation domains; TGF-α, transforming growth factor α; TGF-β3, transforming growth factor beta3; TPT, topotecan; Top I, topoisomerase I; VEGF, vascular endothelial growth factor; bHLH, basic-helix-loop-helix; eIF-4E, eukaryotic translation initiation factor 4E; mTOR, mammalian target of rapamycin; pVHL, von Hippel-Lindau protein.
Figures


Similar articles
-
Cloning, characterization, hypoxia and heat shock response of hypoxia inducible factor-1 (HIF-1) from the small abalone Haliotis diversicolor.Gene. 2014 Jan 25;534(2):256-64. doi: 10.1016/j.gene.2013.10.048. Epub 2013 Nov 7. Gene. 2014. PMID: 24211325
-
Insulin induces transcription of target genes through the hypoxia-inducible factor HIF-1alpha/ARNT.EMBO J. 1998 Sep 1;17(17):5085-94. doi: 10.1093/emboj/17.17.5085. EMBO J. 1998. PMID: 9724644 Free PMC article.
-
Wogonin inhibits tumor angiogenesis via degradation of HIF-1α protein.Toxicol Appl Pharmacol. 2013 Sep 1;271(2):144-55. doi: 10.1016/j.taap.2013.04.031. Epub 2013 May 23. Toxicol Appl Pharmacol. 2013. PMID: 23707765
-
Vasculogenesis and angiogenesis initiation under normoxic conditions through Wnt/β-catenin pathway in gliomas.Rev Neurosci. 2018 Jan 26;29(1):71-91. doi: 10.1515/revneuro-2017-0032. Rev Neurosci. 2018. PMID: 28822229 Review.
-
A systematic review of p53 regulation of oxidative stress in skeletal muscle.Redox Rep. 2018 Dec;23(1):100-117. doi: 10.1080/13510002.2017.1416773. Epub 2018 Jan 3. Redox Rep. 2018. PMID: 29298131 Free PMC article. Review.
Cited by
-
α-Lipoic Acid Maintains Brain Glucose Metabolism via BDNF/TrkB/HIF-1α Signaling Pathway in P301S Mice.Front Aging Neurosci. 2020 Aug 21;12:262. doi: 10.3389/fnagi.2020.00262. eCollection 2020. Front Aging Neurosci. 2020. PMID: 32973490 Free PMC article.
-
Signatures of EMT, immunosuppression, and inflammation in primary and recurrent human cutaneous squamous cell carcinoma at single-cell resolution.Theranostics. 2022 Oct 31;12(17):7532-7549. doi: 10.7150/thno.77528. eCollection 2022. Theranostics. 2022. PMID: 36438481 Free PMC article.
-
Pathologic sequelae of vascular cognitive impairment and dementia sheds light on potential targets for intervention.Cereb Circ Cogn Behav. 2021 Oct 10;2:100030. doi: 10.1016/j.cccb.2021.100030. eCollection 2021. Cereb Circ Cogn Behav. 2021. PMID: 36324710 Free PMC article.
-
Clinical implications of activation of the LIMD1-VHL-HIF1α pathway during head-&-neck squamous cell carcinoma development.Indian J Med Res. 2024 May;159(5):479-493. doi: 10.25259/ijmr_1262_22. Indian J Med Res. 2024. PMID: 39382421 Free PMC article.
-
ACY-241, a histone deacetylase 6 inhibitor, suppresses the epithelial-mesenchymal transition in lung cancer cells by downregulating hypoxia-inducible factor-1 alpha.Korean J Physiol Pharmacol. 2024 Jan 1;28(1):83-91. doi: 10.4196/kjpp.2024.28.1.83. Korean J Physiol Pharmacol. 2024. PMID: 38154967 Free PMC article.
References
-
- Semenza GL. HIF-1 and human disease: one highly involved factor. Genes Dev. 2000;14:1983–1991. - PubMed
-
- Powis G, Kirkpatrick L. Hypoxia inducible factor-1alpha as a cancer drug target. Mol Cancer Ther. 2004;3:647–654. - PubMed
-
- Denko NC. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat Rev Cancer. 2008;8:705–713. - PubMed
-
- Ke QD, Costa M. Hypoxia-inducible factor-1 (HIF-1) Mol Pharmacol. 2006;70:1469–1480. - PubMed
-
- Patiar S, Harris AL. Role of hypoxia-inducible factor-1alpha as a cancer therapy target. Endocr Relat Cancer. 2006;13(Suppl 1):61–75. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

