Posttranslational Modification of HOIP Blocks Toll-Like Receptor 4-Mediated Linear-Ubiquitin-Chain Formation
- PMID: 26578682
- PMCID: PMC4659476
- DOI: 10.1128/mBio.01777-15
Posttranslational Modification of HOIP Blocks Toll-Like Receptor 4-Mediated Linear-Ubiquitin-Chain Formation
Abstract
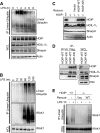
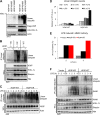
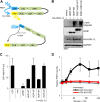
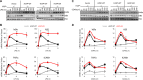
Linear ubiquitination is an atypical posttranslational modification catalyzed by the linear-ubiquitin-chain assembly complex (LUBAC), containing HOIP, HOIL-1L, and Sharpin. LUBAC facilitates NF-κB activation and inflammation upon receptor stimulation by ligating linear ubiquitin chains to critical signaling molecules. Indeed, linear-ubiquitination-dependent signaling is essential to prevent pyogenic bacterial infections that can lead to death. While linear ubiquitination is essential for intracellular receptor signaling upon microbial infection, this response must be measured and stopped to avoid tissue damage and autoimmunity. While LUBAC is activated upon bacterial stimulation, the mechanisms regulating LUBAC activity in response to bacterial stimuli have remained elusive. We demonstrate that LUBAC activity itself is downregulated through ubiquitination, specifically, ubiquitination of the catalytic subunit HOIP at the carboxyl-terminal lysine 1056. Ubiquitination of Lys1056 dynamically altered HOIP conformation, resulting in the suppression of its catalytic activity. Consequently, HOIP Lys1056-to-Arg mutation led not only to persistent LUBAC activity but also to prolonged NF-κB activation induced by bacterial lipopolysaccharide-mediated Toll-like receptor 4 (TLR4) stimulation, whereas it showed no effect on NF-κB activation induced by CD40 stimulation. This study describes a novel posttranslational regulation of LUBAC-mediated linear ubiquitination that is critical for specifically directing TLR4-mediated NF-κB activation.
Importance: Posttranslational modification of proteins enables cells to respond quickly to infections and immune stimuli in a tightly controlled manner. Specifically, covalent modification of proteins with the small protein ubiquitin is essential for cells to initiate and terminate immune signaling in response to bacterial and viral infection. This process is controlled by ubiquitin ligase enzymes, which themselves must be regulated to prevent persistent and deleterious immune signaling. However, how this regulation is achieved is poorly understood. This paper reports a novel ubiquitination event of the atypical ubiquitin ligase HOIP that is required to terminate bacterial lipopolysaccharide (LPS)-induced TLR4 immune signaling. Ubiquitination causes the HOIP ligase to undergo a conformational change, which blocks its enzymatic activity and ultimately terminates LPS-induced TLR4 signaling. These findings provide a new mechanism for controlling HOIP ligase activity that is vital to properly regulate a proinflammatory immune response.
Copyright © 2015 Bowman et al.
Figures


Similar articles
-
Porcine Reproductive and Respiratory Syndrome Virus nsp1α Inhibits NF-κB Activation by Targeting the Linear Ubiquitin Chain Assembly Complex.J Virol. 2017 Jan 18;91(3):e01911-16. doi: 10.1128/JVI.01911-16. Print 2017 Feb 1. J Virol. 2017. PMID: 27881655 Free PMC article.
-
Mechanistic insights into the subversion of the linear ubiquitin chain assembly complex by the E3 ligase IpaH1.4 of Shigella flexneri.Proc Natl Acad Sci U S A. 2022 Mar 22;119(12):e2116776119. doi: 10.1073/pnas.2116776119. Epub 2022 Mar 16. Proc Natl Acad Sci U S A. 2022. PMID: 35294289 Free PMC article.
-
Biochemistry, Pathophysiology, and Regulation of Linear Ubiquitination: Intricate Regulation by Coordinated Functions of the Associated Ligase and Deubiquitinase.Cells. 2021 Oct 9;10(10):2706. doi: 10.3390/cells10102706. Cells. 2021. PMID: 34685685 Free PMC article. Review.
-
The linear ubiquitin assembly complex (LUBAC) is essential for NLRP3 inflammasome activation.J Exp Med. 2014 Jun 30;211(7):1333-47. doi: 10.1084/jem.20132486. Epub 2014 Jun 23. J Exp Med. 2014. PMID: 24958845 Free PMC article.
-
Linear ubiquitination: a novel NF-κB regulatory mechanism for inflammatory and immune responses by the LUBAC ubiquitin ligase complex.Endocr J. 2012;59(8):641-52. doi: 10.1507/endocrj.ej12-0148. Epub 2012 May 19. Endocr J. 2012. PMID: 22673407 Review.
Cited by
-
Acetylation, Phosphorylation, Ubiquitination (Oh My!): Following Post-Translational Modifications on the Ubiquitin Road.Biomolecules. 2022 Mar 18;12(3):467. doi: 10.3390/biom12030467. Biomolecules. 2022. PMID: 35327659 Free PMC article. Review.
-
Role of Linear Ubiquitination in Health and Disease.Am J Respir Cell Mol Biol. 2016 Jun;54(6):761-8. doi: 10.1165/rcmb.2016-0014TR. Am J Respir Cell Mol Biol. 2016. PMID: 26848516 Free PMC article. Review.
-
Transcriptional analyses provide new insight into the late-stage immune response of a diseased Caribbean coral.R Soc Open Sci. 2018 May 16;5(5):172062. doi: 10.1098/rsos.172062. eCollection 2018 May. R Soc Open Sci. 2018. PMID: 29892394 Free PMC article.
-
Ubiquitin-conjugating enzyme UBE2J1 negatively modulates interferon pathway and promotes RNA virus infection.Virol J. 2018 Aug 29;15(1):132. doi: 10.1186/s12985-018-1040-5. Virol J. 2018. PMID: 30157886 Free PMC article.
-
Visualizing ubiquitination in mammalian cells.EMBO Rep. 2019 Feb;20(2):e46520. doi: 10.15252/embr.201846520. Epub 2019 Jan 21. EMBO Rep. 2019. PMID: 30665942 Free PMC article. Review.
References
-
- Boisson B, Laplantine E, Prando C, Giliani S, Israelsson E, Xu Z, Abhyankar A, Israël L, Trevejo-Nunez G, Bogunovic D, Cepika A, MacDuff D, Chrabieh M, Hubeau M, Bajolle F, Debré M, Mazzolari E, Vairo D, Agou F, Virgin HW, Bossuyt X, Rambaud C, Facchetti F, Bonnet D, Quartier P, Fournet JC, Pascual V, Chaussabel D, Notarangelo LD, Puel A, Israel A, Casanova JL, Picard C. 2012. Immunodeficiency, autoinflammation and amylopectinosis in humans with inherited HOIL-1 and LUBAC deficiency. Nat Immunol 13:1178–1186. doi:10.1038/ni.2457. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA197153/CA/NCI NIH HHS/United States
- R01 HL110609/HL/NHLBI NIH HHS/United States
- DE021982/DE/NIDCR NIH HHS/United States
- R01 CA124332/CA/NCI NIH HHS/United States
- R01 AI116585/AI/NIAID NIH HHS/United States
- CA82057/CA/NCI NIH HHS/United States
- AI073099/AI/NIAID NIH HHS/United States
- R01 CA082057/CA/NCI NIH HHS/United States
- R01 DE025465/DE/NIDCR NIH HHS/United States
- CA31363/CA/NCI NIH HHS/United States
- R01 DE023926/DE/NIDCR NIH HHS/United States
- P01 CA180779/CA/NCI NIH HHS/United States
- DE023926/DE/NIDCR NIH HHS/United States
- HL110609/HL/NHLBI NIH HHS/United States
- R01 CA115284/CA/NCI NIH HHS/United States
- AI116585/AI/NIAID NIH HHS/United States
- T90 DE021982/DE/NIDCR NIH HHS/United States
- CA115284/CA/NCI NIH HHS/United States
- R01 AI073099/AI/NIAID NIH HHS/United States
- R01 CA132637/CA/NCI NIH HHS/United States
- R01 CA031363/CA/NCI NIH HHS/United States
- CA180779/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
