Ankyrin-G Inhibits Endocytosis of Cadherin Dimers
- PMID: 26574545
- PMCID: PMC4705390
- DOI: 10.1074/jbc.M115.648386
Ankyrin-G Inhibits Endocytosis of Cadherin Dimers
Abstract
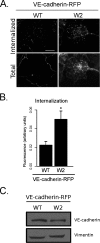
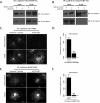
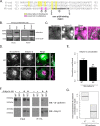
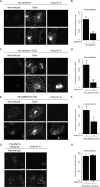
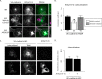
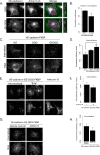
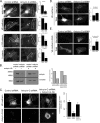
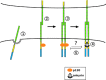
Dynamic regulation of endothelial cell adhesion is central to vascular development and maintenance. Furthermore, altered endothelial adhesion is implicated in numerous diseases. Therefore, normal vascular patterning and maintenance require tight regulation of endothelial cell adhesion dynamics. However, the mechanisms that control junctional plasticity are not fully understood. Vascular endothelial cadherin (VE-cadherin) is an adhesive protein found in adherens junctions of endothelial cells. VE-cadherin mediates adhesion through trans interactions formed by its extracellular domain. Trans binding is followed by cis interactions that laterally cluster the cadherin in junctions. VE-cadherin is linked to the actin cytoskeleton through cytoplasmic interactions with β- and α-catenin, which serve to increase adhesive strength. Furthermore, p120-catenin binds to the cytoplasmic tail of cadherin and stabilizes it at the plasma membrane. Here we report that induced cis dimerization of VE-cadherin inhibits endocytosis independent of both p120 binding and trans interactions. However, we find that ankyrin-G, a protein that links membrane proteins to the spectrin-actin cytoskeleton, associates with VE-cadherin and inhibits its endocytosis. Ankyrin-G inhibits VE-cadherin endocytosis independent of p120 binding. We propose a model in which ankyrin-G associates with and inhibits the endocytosis of VE-cadherin cis dimers. Our findings support a novel mechanism for regulation of VE-cadherin endocytosis through ankyrin association with cadherin engaged in lateral interactions.
Keywords: adaptor protein; adherens junction; adhesion; cadherin; endocytosis; endothelial cell.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

Similar articles
-
p120-catenin binding masks an endocytic signal conserved in classical cadherins.J Cell Biol. 2012 Oct 15;199(2):365-80. doi: 10.1083/jcb.201205029. J Cell Biol. 2012. PMID: 23071156 Free PMC article.
-
p120-catenin and β-catenin differentially regulate cadherin adhesive function.Mol Biol Cell. 2013 Mar;24(6):704-14. doi: 10.1091/mbc.E12-06-0471. Epub 2013 Jan 16. Mol Biol Cell. 2013. PMID: 23325790 Free PMC article.
-
p120-catenin regulates VE-cadherin endocytosis and degradation induced by the Kaposi sarcoma-associated ubiquitin ligase K5.Mol Biol Cell. 2017 Jan 1;28(1):30-40. doi: 10.1091/mbc.E16-06-0459. Epub 2016 Oct 26. Mol Biol Cell. 2017. PMID: 27798235 Free PMC article.
-
Dynamic Regulation of Vascular Permeability by Vascular Endothelial Cadherin-Mediated Endothelial Cell-Cell Junctions.J Nippon Med Sch. 2017;84(4):148-159. doi: 10.1272/jnms.84.148. J Nippon Med Sch. 2017. PMID: 28978894 Review.
-
Structure of artificial and natural VE-cadherin-based adherens junctions.Biochem Soc Trans. 2008 Apr;36(Pt 2):189-93. doi: 10.1042/BST0360189. Biochem Soc Trans. 2008. PMID: 18363560 Free PMC article. Review.
Cited by
-
Ankyrin G organizes membrane components to promote coupling of cell mechanics and glucose uptake.Nat Cell Biol. 2021 May;23(5):457-466. doi: 10.1038/s41556-021-00677-y. Epub 2021 May 10. Nat Cell Biol. 2021. PMID: 33972734 Free PMC article.
-
Feedback-Driven Assembly of the Axon Initial Segment.Neuron. 2019 Oct 23;104(2):305-321.e8. doi: 10.1016/j.neuron.2019.07.029. Epub 2019 Aug 29. Neuron. 2019. PMID: 31474508 Free PMC article.
-
Ankrd31 in Sperm and Epididymal Integrity.Front Cell Dev Biol. 2021 Nov 8;9:741975. doi: 10.3389/fcell.2021.741975. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34820371 Free PMC article.
-
The VE-cadherin cytoplasmic domain undergoes proteolytic processing during endocytosis.Mol Biol Cell. 2017 Jan 1;28(1):76-84. doi: 10.1091/mbc.E16-09-0658. Epub 2016 Oct 26. Mol Biol Cell. 2017. PMID: 27798242 Free PMC article.
-
Analysis of Combined Transcriptomes Identifies Gene Modules that Differentially Respond to Pathogenic Stimulation of Vascular Smooth Muscle and Endothelial Cells.Sci Rep. 2018 Jan 10;8(1):395. doi: 10.1038/s41598-017-18675-2. Sci Rep. 2018. PMID: 29321689 Free PMC article.
References
-
- Carmeliet P. (2003) Angiogenesis in health and disease. Nat. Med. 9, 653–660 - PubMed
-
- Dejana E., Orsenigo F., and Lampugnani M. G. (2008) The role of adherens junctions and VE-cadherin in the control of vascular permeability. J. Cell Sci. 121, 2115–2122 - PubMed
-
- Vincent P. A., Xiao K., Buckley K. M., and Kowalczyk A. P. (2004) VE-cadherin: adhesion at arm's length. Am. J. Physiol. Cell Physiol. 286, C987–997 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

