Mouse Resistin (mRetn): cloning, expression and purification in Escherichia coli and the potential regulative effects on murine bone marrow hematopoiesis
- PMID: 26572487
- PMCID: PMC4647653
- DOI: 10.1186/s12896-015-0221-1
Mouse Resistin (mRetn): cloning, expression and purification in Escherichia coli and the potential regulative effects on murine bone marrow hematopoiesis
Abstract
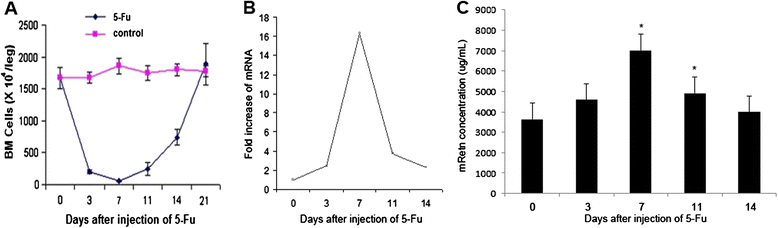
Background: Resistin (Retn) is a cytokine which has a controversial physiological role regarding its involvement with obesity and type II diabetes mellitus. Recently, murine Retn was found to be a possibly potential regulator of hematopoiesis in mice shown in the screening results of a set of gene chips which mapped the expression level of murine genes during regeneration of impaired bone marrow (BM) by 5-fluorouracil.
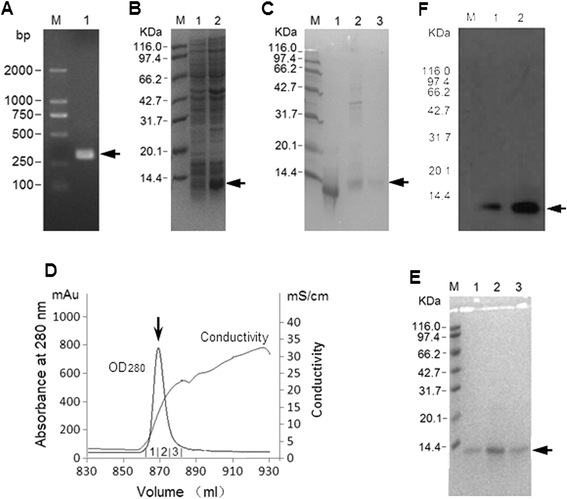
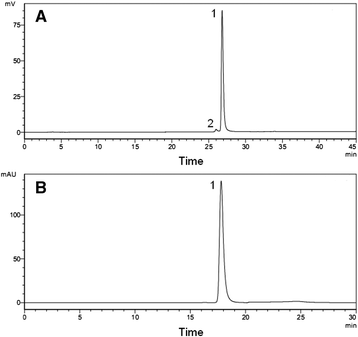
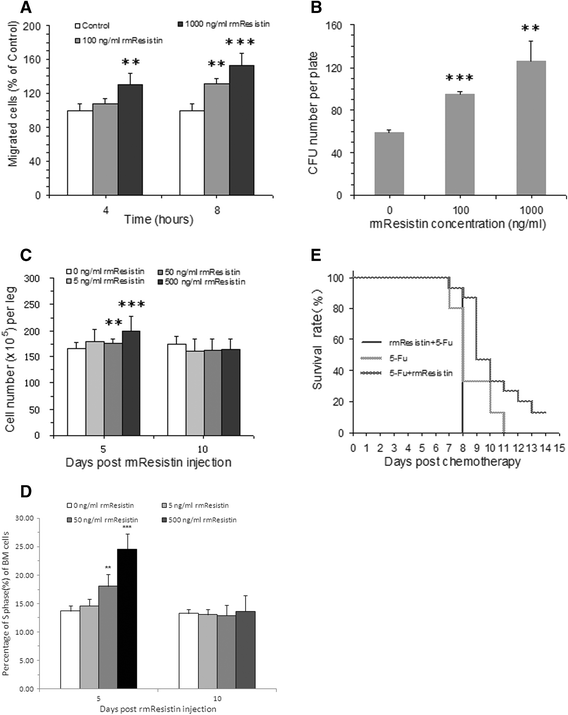
Results: Recombinant mice Retn was expressed in Escherichia coli and purified using ion exchange chromatography. Totally 11.4 mg rmRetn was obtained from 500 ml culture with endotoxin level less than 1.0 EU/ug. The purity of recombinant murine Resistin reached to at least 97.6% via SDS-PAGE analysis and HPLC. The protein possessed chemotaxis effects in the mouse aortic endothelial cells in vitro in transwell analysis. In vitro, rmRetn could up regulate the CFU number of mice BM and after rmRetn was administered, the cell number of murine bone marrow was significantly increased in vivo after chemotherapy. Finally, rmRetn was found able to protect mice from the chemotoxicity of 5-fluorouracil.
Conclusions: The discovery demonstrated a new function of murine Retn and suggested that it could potentially accelerate bone marrow regeneration post chemotherapy.
Figures
Similar articles
-
Production and characterization of recombinant human and murine TNF.Methods Mol Med. 2004;98:9-22. doi: 10.1385/1-59259-771-8:009. Methods Mol Med. 2004. PMID: 15064429
-
Human resistin is a systemic immune-derived proinflammatory cytokine targeting both leukocytes and adipocytes.PLoS One. 2006 Dec 20;1(1):e31. doi: 10.1371/journal.pone.0000031. PLoS One. 2006. PMID: 17183659 Free PMC article.
-
Echinacoside improves hematopoietic function in 5-FU-induced myelosuppression mice.Life Sci. 2015 Feb 15;123:86-92. doi: 10.1016/j.lfs.2015.01.002. Epub 2015 Jan 23. Life Sci. 2015. PMID: 25623854
-
Inflammatory induction of human resistin causes insulin resistance in endotoxemic mice.Diabetes. 2011 Mar;60(3):775-83. doi: 10.2337/db10-1416. Epub 2011 Jan 31. Diabetes. 2011. PMID: 21282361 Free PMC article.
-
Role of hematopoietic microenvironment in prolonged impairment of B cell regeneration in age-related stromal-cell-impaired SAMP1 mouse: effects of a single dose of 5-fluorouracil.J Appl Toxicol. 2008 Aug;28(6):797-805. doi: 10.1002/jat.1341. J Appl Toxicol. 2008. PMID: 18344199
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

