Live-Attenuated Bacterial Vectors: Tools for Vaccine and Therapeutic Agent Delivery
- PMID: 26569321
- PMCID: PMC4693226
- DOI: 10.3390/vaccines3040940
Live-Attenuated Bacterial Vectors: Tools for Vaccine and Therapeutic Agent Delivery
Abstract
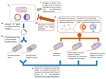
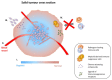
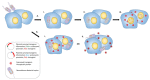
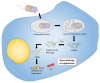
Genetically attenuated microorganisms, including pathogenic and commensal bacteria, can be engineered to carry and deliver heterologous antigens to elicit host immunity against both the vector as well as the pathogen from which the donor gene is derived. These live attenuated bacterial vectors have been given much attention due to their capacity to induce a broad range of immune responses including localized mucosal, as well as systemic humoral and/or cell-mediated immunity. In addition, the unique tumor-homing characteristics of these bacterial vectors has also been exploited for alternative anti-tumor vaccines and therapies. In such approach, tumor-associated antigen, immunostimulatory molecules, anti-tumor drugs, or nucleotides (DNA or RNA) are delivered. Different potential vectors are appropriate for specific applications, depending on their pathogenic routes. In this review, we survey and summarize the main features of the different types of live bacterial vectors and discussed the clinical applications in the field of vaccinology. In addition, different approaches for using live attenuated bacterial vectors for anti-cancer therapy is discussed, and some promising pre-clinical and clinical studies in this field are outlined.
Keywords: Salmonella; attenuated vector; cancer; infectious disease; vaccine.
Figures
Similar articles
-
Live bacterial vaccine vectors: an overview.Braz J Microbiol. 2015 Mar 4;45(4):1117-29. doi: 10.1590/s1517-83822014000400001. eCollection 2014. Braz J Microbiol. 2015. PMID: 25763014 Free PMC article. Review.
-
Live bacterial vaccine vector and delivery strategies of heterologous antigen: A review.Immunol Lett. 2018 May;197:70-77. doi: 10.1016/j.imlet.2018.03.006. Epub 2018 Mar 14. Immunol Lett. 2018. PMID: 29550258 Review.
-
[Therapeutic intervention alternatives in cancer, using attenuated live bacterial vectors: Salmonella enterica as a carrier of heterologous molecules].Rev Invest Clin. 2013 Jan-Feb;65(1):65-73. Rev Invest Clin. 2013. PMID: 23745445 Review. Spanish.
-
Live recombinant vectors for AIDS vaccine development.Curr Mol Med. 2003 May;3(3):273-84. doi: 10.2174/1566524033479816. Curr Mol Med. 2003. PMID: 12699363 Review.
-
Live, attenuated strains of Listeria and Salmonella as vaccine vectors in cancer treatment.Bioeng Bugs. 2010 Jul-Aug;1(4):235-43. doi: 10.4161/bbug.1.4.11243. Epub 2010 Jan 4. Bioeng Bugs. 2010. PMID: 21327055 Free PMC article. Review.
Cited by
-
Advances in Next-Generation Coronavirus Vaccines in Response to Future Virus Evolution.Vaccines (Basel). 2022 Nov 29;10(12):2035. doi: 10.3390/vaccines10122035. Vaccines (Basel). 2022. PMID: 36560445 Free PMC article. Review.
-
Bacteria-Based Nanoprobes for Cancer Therapy.Int J Nanomedicine. 2024 Jan 23;19:759-785. doi: 10.2147/IJN.S438164. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38283198 Free PMC article. Review.
-
Mucosal Vaccine Approaches for Prevention of HIV and SIV Transmission.Curr Immunol Rev. 2019;15(1):102-122. doi: 10.2174/1573395514666180605092054. Curr Immunol Rev. 2019. PMID: 31452652 Free PMC article.
-
Cancer Vaccines: From the State of the Art to the Most Promising Frontiers in the Treatment of Colorectal Cancer.Pharmaceutics. 2023 Jul 17;15(7):1969. doi: 10.3390/pharmaceutics15071969. Pharmaceutics. 2023. PMID: 37514155 Free PMC article. Review.
-
Targeting Melanoma Hypoxia with the Food-Grade Lactic Acid Bacterium Lactococcus Lactis.Cancers (Basel). 2020 Feb 13;12(2):438. doi: 10.3390/cancers12020438. Cancers (Basel). 2020. PMID: 32069844 Free PMC article.
References
-
- Van T.T.H., Lin Y.-C., Van T.N.N., Nguyen T.Q., Le T.T.H., Do T.H., Truong N.H., Coloe P.J., Smooker P.M. Salmonella as a vaccine vector for influenza virus. Procedia Vaccinol. 2013;7:23–27. doi: 10.1016/j.provac.2013.06.005. - DOI
-
- Goya A.K., Khatri K., Mishra N., Vyas S.P. New patents on mucosal delivery of vaccines. Expert Opin. Ther. Pat. 2008;18:1271–1288.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

