Fluid shear triggers microvilli formation via mechanosensitive activation of TRPV6
- PMID: 26563429
- PMCID: PMC4660203
- DOI: 10.1038/ncomms9871
Fluid shear triggers microvilli formation via mechanosensitive activation of TRPV6
Abstract
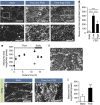
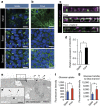
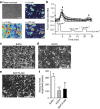
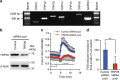
Microvilli are cellular membrane protrusions present on differentiated epithelial cells, which can sense and interact with the surrounding fluid environment. Biochemical and genetic approaches have identified a set of factors involved in microvilli formation; however, the underlying extrinsic regulatory mechanism of microvilli formation remains largely unknown. Here we demonstrate that fluid shear stress (FSS), an external mechanical cue, serves as a trigger for microvilli formation in human placental trophoblastic cells. We further reveal that the transient receptor potential, vanilloid family type-6 (TRPV6) calcium ion channel plays a critical role in flow-induced Ca(2+) influx and microvilli formation. TRPV6 regulates phosphorylation of Ezrin via a Ca(2+)-dependent phosphorylation of Akt; this molecular event is necessary for microvillar localization of Ezrin in response to FSS. Our findings provide molecular insight into the microvilli-mediated mechanoresponsive cellular functions, such as epithelial absorption, signal perception and mechanotransduction.
Figures

Similar articles
-
Maternal Transient Receptor Potential Vanilloid 6 (Trpv6) Is Involved In Offspring Bone Development.J Bone Miner Res. 2019 Apr;34(4):699-710. doi: 10.1002/jbmr.3646. Epub 2019 Feb 20. J Bone Miner Res. 2019. PMID: 30786075
-
Sleep Deprivation Induces Dry Eye Through Inhibition of PPARα Expression in Corneal Epithelium.Invest Ophthalmol Vis Sci. 2018 Nov 1;59(13):5494-5508. doi: 10.1167/iovs.18-24504. Invest Ophthalmol Vis Sci. 2018. PMID: 30658033
-
G protein-coupled receptor signaling via Src kinase induces endogenous human transient receptor potential vanilloid type 6 (TRPV6) channel activation.J Biol Chem. 2011 Apr 15;286(15):13184-92. doi: 10.1074/jbc.M110.183525. Epub 2011 Feb 24. J Biol Chem. 2011. PMID: 21349844 Free PMC article.
-
Human TRPV6-pathies caused by gene mutations.Biochim Biophys Acta Gen Subj. 2021 Jun;1865(6):129873. doi: 10.1016/j.bbagen.2021.129873. Epub 2021 Feb 19. Biochim Biophys Acta Gen Subj. 2021. PMID: 33610740 Review.
-
Advances in TRPV6 inhibitors for tumors by targeted therapies: Macromolecular proteins, synthetic small molecule compounds, and natural compounds.Eur J Med Chem. 2024 Apr 15;270:116379. doi: 10.1016/j.ejmech.2024.116379. Epub 2024 Apr 1. Eur J Med Chem. 2024. PMID: 38588625 Review.
Cited by
-
Building the brush border, one microvillus at a time.Curr Opin Cell Biol. 2023 Feb;80:102153. doi: 10.1016/j.ceb.2023.102153. Epub 2023 Feb 22. Curr Opin Cell Biol. 2023. PMID: 36827850 Free PMC article. Review.
-
Trophoblast organoids with physiological polarity model placental structure and function.bioRxiv [Preprint]. 2023 Jul 17:2023.01.12.523752. doi: 10.1101/2023.01.12.523752. bioRxiv. 2023. Update in: J Cell Sci. 2024 Mar 1;137(5):jcs261528. doi: 10.1242/jcs.261528. PMID: 36711688 Free PMC article. Updated. Preprint.
-
Biosensors for Detection of Human Placental Pathologies: A Review of Emerging Technologies and Current Trends.Transl Res. 2019 Nov;213:23-49. doi: 10.1016/j.trsl.2019.05.002. Epub 2019 May 20. Transl Res. 2019. PMID: 31170377 Free PMC article. Review.
-
Development of a human iPSC-derived placental barrier-on-chip model.iScience. 2023 Jul 13;26(7):107240. doi: 10.1016/j.isci.2023.107240. eCollection 2023 Jul 21. iScience. 2023. PMID: 37534160 Free PMC article.
-
Collagen VI Deficiency Impairs Tendon Fibroblasts Mechanoresponse in Ullrich Congenital Muscular Dystrophy.Cells. 2024 Feb 22;13(5):378. doi: 10.3390/cells13050378. Cells. 2024. PMID: 38474342 Free PMC article.
References
-
- Kenny A. J. & Maroux S. Topology of microvillar membrance hydrolases of kidney and intestine. Physiol. Rev. 62, 91–128 (1982). - PubMed
-
- Lange K. Fundamental role of microvilli in the main functions of differentiated cells: outline of an universal regulating and signaling system at the cell periphery. J. Cell. Physiol. 226, 896–927 (2011). - PubMed
-
- Al-Zuhair A. G., Ibrahim M. E., Mughal S. & Abdulla M. A. Loss and regeneration of the microvilli of human placental syncytiotrophoblast. Arch. Gynecol. 240, 147–151 (1987). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

