Umbilical cord blood-derived T regulatory cells to prevent GVHD: kinetics, toxicity profile, and clinical effect
- PMID: 26563133
- PMCID: PMC4768428
- DOI: 10.1182/blood-2015-06-653667
Umbilical cord blood-derived T regulatory cells to prevent GVHD: kinetics, toxicity profile, and clinical effect
Abstract
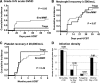
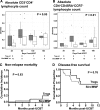
We studied the safety and clinical outcomes of patients treated with umbilical cord blood (UCB)-derived regulatory T cells (Tregs) that expanded in cultures stimulated with K562 cells modified to express the high-affinity Fc receptor (CD64) and CD86, the natural ligand of CD28 (KT64/86). Eleven patients were treated with Treg doses from 3-100 × 10(6) Treg/kg. The median proportion of CD4(+)FoxP3(+)CD127(-) in the infused product was 87% (range, 78%-95%), and we observed no dose-limiting infusional adverse events. Clinical outcomes were compared with contemporary controls (n = 22) who received the same conditioning regimen with sirolimus and mycophenolate mofetil immune suppression. The incidence of grade II-IV acute graft-versus-host disease (GVHD) at 100 days was 9% (95% confidence interval [CI], 0-25) vs 45% (95% CI, 24-67) in controls (P = .05). Chronic GVHD at 1 year was zero in Tregs and 14% in controls. Hematopoietic recovery and chimerism, cumulative density of infections, nonrelapse mortality, relapse, and disease-free survival were similar in the Treg recipients and controls. KT64/86-expanded UCB Tregs were safe and resulted in low risk of acute GVHD.
© 2016 by The American Society of Hematology.
Figures

Comment in
-
Treg adoptive therapy: is more better?Blood. 2016 Feb 25;127(8):962-3. doi: 10.1182/blood-2015-12-682492. Blood. 2016. PMID: 26917738 No abstract available.
Similar articles
-
Sirolimus and Mycophenolate Mofetil as Calcineurin Inhibitor-Free Graft-versus-Host Disease Prophylaxis for Reduced-Intensity Conditioning Umbilical Cord Blood Transplantation.Biol Blood Marrow Transplant. 2016 Nov;22(11):2025-2030. doi: 10.1016/j.bbmt.2016.08.005. Epub 2016 Aug 9. Biol Blood Marrow Transplant. 2016. PMID: 27519278 Free PMC article. Clinical Trial.
-
Phase I/II Study of Stem-Cell Transplantation Using a Single Cord Blood Unit Expanded Ex Vivo With Nicotinamide.J Clin Oncol. 2019 Feb 10;37(5):367-374. doi: 10.1200/JCO.18.00053. Epub 2018 Dec 4. J Clin Oncol. 2019. PMID: 30523748 Free PMC article. Clinical Trial.
-
Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics.Blood. 2011 Jan 20;117(3):1061-70. doi: 10.1182/blood-2010-07-293795. Epub 2010 Oct 15. Blood. 2011. PMID: 20952687 Free PMC article. Clinical Trial.
-
Alloreactivity as therapeutic principle in the treatment of hematologic malignancies. Studies of clinical and immunologic aspects of allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning.Dan Med Bull. 2007 May;54(2):112-39. Dan Med Bull. 2007. PMID: 17521527 Review.
-
Immune regulatory cells in umbilical cord blood: T regulatory cells and mesenchymal stromal cells.Br J Haematol. 2009 Oct;147(2):200-6. doi: 10.1111/j.1365-2141.2009.07781.x. Br J Haematol. 2009. PMID: 19796269 Free PMC article. Review.
Cited by
-
Beyond CAR-T: The rise of CAR-NK cell therapy in asthma immunotherapy.J Transl Med. 2024 Aug 5;22(1):736. doi: 10.1186/s12967-024-05534-8. J Transl Med. 2024. PMID: 39103889 Free PMC article. Review.
-
Peri-alloHCT IL-33 administration expands recipient T-regulatory cells that protect mice against acute GVHD.Blood. 2016 Jul 21;128(3):427-39. doi: 10.1182/blood-2015-12-684142. Epub 2016 May 24. Blood. 2016. PMID: 27222477 Free PMC article.
-
Mesenchymal stem cells transfer mitochondria to allogeneic Tregs in an HLA-dependent manner improving their immunosuppressive activity.Nat Commun. 2022 Feb 14;13(1):856. doi: 10.1038/s41467-022-28338-0. Nat Commun. 2022. PMID: 35165293 Free PMC article.
-
Graft Engineering and Adoptive Immunotherapy: New Approaches to Promote Immune Tolerance After Hematopoietic Stem Cell Transplantation.Front Immunol. 2019 Jul 10;10:1342. doi: 10.3389/fimmu.2019.01342. eCollection 2019. Front Immunol. 2019. PMID: 31354695 Free PMC article. Review.
-
A phase 1 study of donor regulatory T-cell infusion plus low-dose interleukin-2 for steroid-refractory chronic graft-vs-host disease.Blood Adv. 2022 Nov 8;6(21):5786-5796. doi: 10.1182/bloodadvances.2021006625. Blood Adv. 2022. PMID: 35475885 Free PMC article. Clinical Trial.
References
-
- Sakaguchi S. Regulatory T cells: history and perspective. Methods Mol Biol. 2011;707:3–17. - PubMed
-
- Shen LS, Wang J, Shen DF, et al. CD4(+)CD25(+)CD127(low/-) regulatory T cells express Foxp3 and suppress effector T cell proliferation and contribute to gastric cancers progression. Clin Immunol. 2009;131(1):109–118. - PubMed
-
- Yu N, Li X, Song W, et al. CD4(+)CD25 (+)CD127 (low/-) T cells: a more specific Treg population in human peripheral blood. Inflammation. 2012;35(6):1773–1780. - PubMed
Publication types
MeSH terms
Grants and funding
- P01 CA65493/CA/NCI NIH HHS/United States
- P30 CA77598/CA/NCI NIH HHS/United States
- R01 CA105216/CA/NCI NIH HHS/United States
- P30 CA077598/CA/NCI NIH HHS/United States
- R01 HL118979/HL/NHLBI NIH HHS/United States
- R01 HL11879/HL/NHLBI NIH HHS/United States
- R01 AI034495/AI/NIAID NIH HHS/United States
- P30 CA016520/CA/NCI NIH HHS/United States
- R01 HL056067/HL/NHLBI NIH HHS/United States
- R01 CA072669/CA/NCI NIH HHS/United States
- UL1 TR002494/TR/NCATS NIH HHS/United States
- P01 CA065493/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

