Oncolytic Replication of E1b-Deleted Adenoviruses
- PMID: 26561828
- PMCID: PMC4664978
- DOI: 10.3390/v7112905
Oncolytic Replication of E1b-Deleted Adenoviruses
Abstract
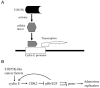
Various viruses have been studied and developed for oncolytic virotherapies. In virotherapy, a relatively small amount of viruses used in an intratumoral injection preferentially replicate in and lyse cancer cells, leading to the release of amplified viral particles that spread the infection to the surrounding tumor cells and reduce the tumor mass. Adenoviruses (Ads) are most commonly used for oncolytic virotherapy due to their infection efficacy, high titer production, safety, easy genetic modification, and well-studied replication characteristics. Ads with deletion of E1b55K preferentially replicate in and destroy cancer cells and have been used in multiple clinical trials. H101, one of the E1b55K-deleted Ads, has been used for the treatment of late-stage cancers as the first approved virotherapy agent. However, the mechanism of selective replication of E1b-deleted Ads in cancer cells is still not well characterized. This review will focus on three potential molecular mechanisms of oncolytic replication of E1b55K-deleted Ads. These mechanisms are based upon the functions of the viral E1B55K protein that are associated with p53 inhibition, late viralmRNAexport, and cell cycle disruption.
Keywords: E1B; adenovirus; cancer selectivity; cell cycle; cyclin E; virotherapy.
Figures

Similar articles
-
Functional interactions of antiapoptotic proteins and tumor necrosis factor in the context of a replication-competent adenovirus.Gene Ther. 2005 Sep;12(17):1333-46. doi: 10.1038/sj.gt.3302555. Gene Ther. 2005. PMID: 15920462
-
Oncolytic E1B 55KDa-deleted adenovirus replication is independent of p53 levels in cancer cells.Cell Mol Biol (Noisy-le-grand). 2017 Aug 15;63(7):1-11. doi: 10.14715/cmb/2017.63.7.1. Cell Mol Biol (Noisy-le-grand). 2017. PMID: 28838332
-
E1A, E1B double-restricted adenovirus with RGD-fiber modification exhibits enhanced oncolysis for CAR-deficient biliary cancers.Clin Cancer Res. 2007 May 15;13(10):3043-50. doi: 10.1158/1078-0432.CCR-06-2103. Clin Cancer Res. 2007. PMID: 17505007
-
Recent advances in oncolytic adenovirus therapies for cancer.Curr Opin Virol. 2016 Dec;21:9-15. doi: 10.1016/j.coviro.2016.06.009. Epub 2016 Jul 2. Curr Opin Virol. 2016. PMID: 27379906 Free PMC article. Review.
-
Clinical trials with oncolytic adenovirus in China.Curr Cancer Drug Targets. 2007 Mar;7(2):141-8. doi: 10.2174/156800907780058817. Curr Cancer Drug Targets. 2007. PMID: 17346105 Review.
Cited by
-
New hopes for the breast cancer treatment: perspectives on the oncolytic virus therapy.Front Immunol. 2024 Mar 21;15:1375433. doi: 10.3389/fimmu.2024.1375433. eCollection 2024. Front Immunol. 2024. PMID: 38576614 Free PMC article. Review.
-
From Benchtop to Bedside: A Review of Oncolytic Virotherapy.Biomedicines. 2016 Aug 2;4(3):18. doi: 10.3390/biomedicines4030018. Biomedicines. 2016. PMID: 28536385 Free PMC article. Review.
-
Development and application of oncolytic viruses as the nemesis of tumor cells.Front Microbiol. 2023 Jun 12;14:1188526. doi: 10.3389/fmicb.2023.1188526. eCollection 2023. Front Microbiol. 2023. PMID: 37440883 Free PMC article. Review.
-
Adenovirus Receptor Expression in Cancer and Its Multifaceted Role in Oncolytic Adenovirus Therapy.Int J Mol Sci. 2020 Sep 17;21(18):6828. doi: 10.3390/ijms21186828. Int J Mol Sci. 2020. PMID: 32957644 Free PMC article. Review.
-
Immunotherapeutic Strategies for the Treatment of Glioblastoma: Current Challenges and Future Perspectives.Cancers (Basel). 2024 Mar 25;16(7):1276. doi: 10.3390/cancers16071276. Cancers (Basel). 2024. PMID: 38610954 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

