Murine versus human apolipoprotein E4: differential facilitation of and co-localization in cerebral amyloid angiopathy and amyloid plaques in APP transgenic mouse models
- PMID: 26556230
- PMCID: PMC4641345
- DOI: 10.1186/s40478-015-0250-y
Murine versus human apolipoprotein E4: differential facilitation of and co-localization in cerebral amyloid angiopathy and amyloid plaques in APP transgenic mouse models
Abstract
Introduction: Amyloid β (Aβ) accumulates in the extracellular space as diffuse and neuritic plaques in Alzheimer's disease (AD). Aβ also deposits on the walls of arterioles as cerebral amyloid angiopathy (CAA) in most cases of AD and sometimes independently of AD. Apolipoprotein E (apoE) ɛ4 is associated with increases in both Aβ plaques and CAA in humans. Studies in mouse models that develop Aβ deposition have shown that murine apoE and human apoE4 have different abilities to facilitate plaque or CAA formation when studied independently. To better understand and compare the effects of murine apoE and human apoE4, we bred 5XFAD (line 7031) transgenic mice so that they expressed one copy of murine apoE and one copy of human apoE4 under the control of the normal murine apoE regulatory elements (5XFAD/apoE(m/4)).
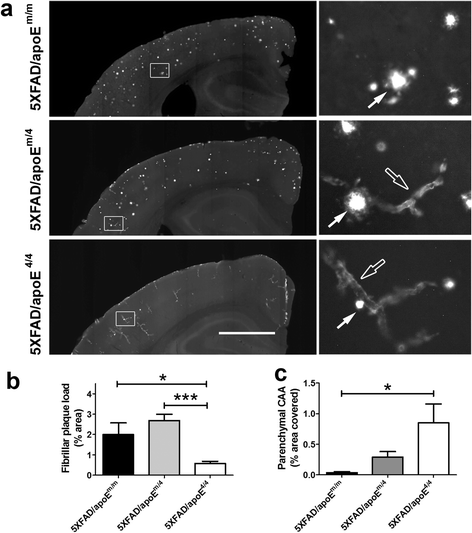
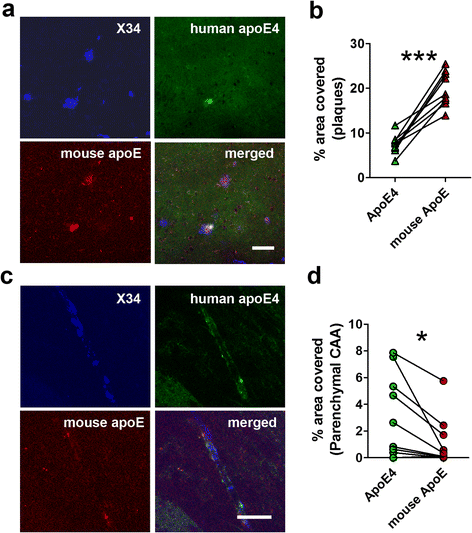
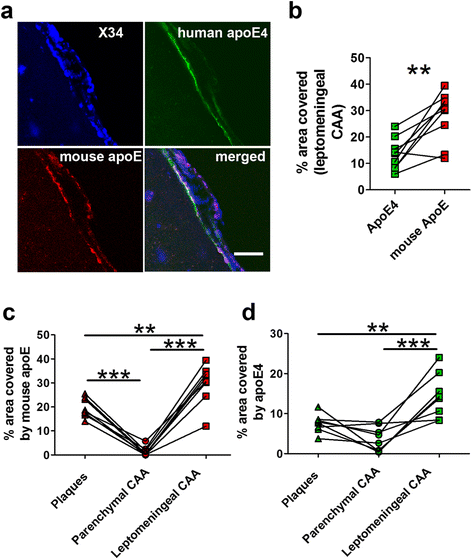
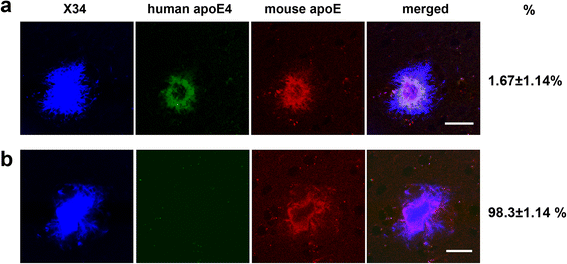
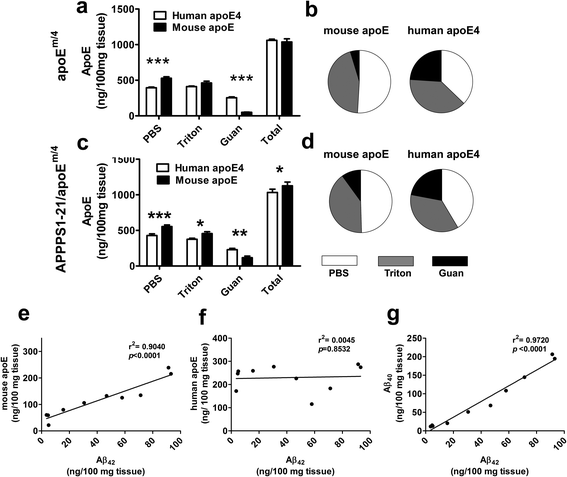
Results: The 5XFAD/apoE(m/4) mice contained levels of parenchymal CAA that were intermediate between 5XFAD/apoE(m/m) and 5XFAD/apoE(4/4) mice. In 5XFAD/apoE(m/4) mice, we found that Aβ parenchymal plaques co-localized with much more apoE than did parenchymal CAA, suggesting differential co-aggregation of apoE with Aβ in plaques versus CAA. More importantly, within the brain parenchyma of the 5XFAD/apoE(m/4) mice, plaques contained more murine apoE, which on its own results in more pronounced and earlier plaque formation, while CAA contained more human apoE4 which on its own results in more pronounced CAA formation. We further confirmed the co-aggregation of mouse apoE with Aβ in plaques by showing a strong correlation between insoluble mouse apoE and insoluble Aβ in PS1APP-21/apoE(m/4) mice which develop plaques without CAA.
Conclusions: These studies suggest that both murine apoE and human apoE4 facilitate differential opposing effects in influencing Aβ plaques versus CAA via different co-aggregation with these two amyloid lesions and set the stage for understanding these effects at a molecular level.
Figures
Similar articles
-
Human apolipoprotein E4 alters the amyloid-beta 40:42 ratio and promotes the formation of cerebral amyloid angiopathy in an amyloid precursor protein transgenic model.J Neurosci. 2005 Mar 16;25(11):2803-10. doi: 10.1523/JNEUROSCI.5170-04.2005. J Neurosci. 2005. PMID: 15772340 Free PMC article.
-
Astrocytic APOE4 removal confers cerebrovascular protection despite increased cerebral amyloid angiopathy.Mol Neurodegener. 2023 Mar 16;18(1):17. doi: 10.1186/s13024-023-00610-x. Mol Neurodegener. 2023. PMID: 36922879 Free PMC article.
-
Human apolipoprotein E2 promotes parenchymal amyloid deposition and neuronal loss in vasculotropic mutant amyloid-β protein Tg-SwDI mice.J Alzheimers Dis. 2012;31(2):359-69. doi: 10.3233/JAD-2012-120421. J Alzheimers Dis. 2012. PMID: 22635103
-
Role of apoe/Abeta interactions in the pathogenesis of Alzheimer's disease and cerebral amyloid angiopathy.J Mol Neurosci. 2001 Oct;17(2):147-55. doi: 10.1385/JMN:17:2:147. J Mol Neurosci. 2001. PMID: 11816788 Review.
-
Hereditary and sporadic forms of abeta-cerebrovascular amyloidosis and relevant transgenic mouse models.Int J Mol Sci. 2009 Apr 23;10(4):1872-1895. doi: 10.3390/ijms10041872. Int J Mol Sci. 2009. PMID: 19468344 Free PMC article. Review.
Cited by
-
Standardized immunoprecipitation protocol for efficient isolation of native apolipoprotein E particles utilizing HJ15.4 monoclonal antibody.STAR Protoc. 2023 Jun 7;4(2):102271. doi: 10.1016/j.xpro.2023.102271. Online ahead of print. STAR Protoc. 2023. PMID: 37289593 Free PMC article.
-
Modeling sporadic Alzheimer's disease in mice by combining Apolipoprotein E4 risk gene with environmental risk factors.Front Aging Neurosci. 2024 Feb 27;16:1357405. doi: 10.3389/fnagi.2024.1357405. eCollection 2024. Front Aging Neurosci. 2024. PMID: 38476659 Free PMC article.
-
Peripheral versus central nervous system APOE in Alzheimer's disease: Interplay across the blood-brain barrier.Neurosci Lett. 2019 Aug 24;708:134306. doi: 10.1016/j.neulet.2019.134306. Epub 2019 Jun 7. Neurosci Lett. 2019. PMID: 31181302 Free PMC article. Review.
-
A1 reactive astrocytes and a loss of TREM2 are associated with an early stage of pathology in a mouse model of cerebral amyloid angiopathy.J Neuroinflammation. 2020 Jul 25;17(1):223. doi: 10.1186/s12974-020-01900-7. J Neuroinflammation. 2020. PMID: 32711525 Free PMC article.
-
Selective reduction of astrocyte apoE3 and apoE4 strongly reduces Aβ accumulation and plaque-related pathology in a mouse model of amyloidosis.Mol Neurodegener. 2022 Feb 2;17(1):13. doi: 10.1186/s13024-022-00516-0. Mol Neurodegener. 2022. PMID: 35109920 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

