Development of Budesonide Loaded Biopolymer Based Dry Powder Inhaler: Optimization, In Vitro Deposition, and Cytotoxicity Study
- PMID: 26556201
- PMCID: PMC4590799
- DOI: 10.1155/2014/795371
Development of Budesonide Loaded Biopolymer Based Dry Powder Inhaler: Optimization, In Vitro Deposition, and Cytotoxicity Study
Abstract
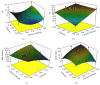
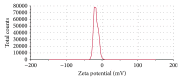
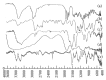
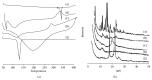
The progress in the development of DPI technology has boosted the use of sensitive drug molecules for lung diseases. However, delivery of these molecules from conventional DPI to the active site still poses a challenge with respect to deposition efficiency in the lung. At same time, serious systemic side effects of drugs have become a cause for concern. The developed budesonide loaded biopolymer based controlled release DPI had shown maximum in vitro lung deposition with least toxicity. The subject of present study, lactose-free budesonide loaded biopolymer based DPI, further corroborates the great potential of antiasthmatic drugs. This technology is expected to revolutionize the approaches towards enhanced therapeutic delivery of prospective drugs.
Figures

Similar articles
-
Dry-Powder Inhaler Formulation of Rifampicin: An Improved Targeted Delivery System for Alveolar Tuberculosis.J Aerosol Med Pulm Drug Deliv. 2017 Dec;30(6):388-398. doi: 10.1089/jamp.2017.1379. Epub 2017 May 16. J Aerosol Med Pulm Drug Deliv. 2017. PMID: 28510480
-
Computationally efficient analysis of particle transport and deposition in a human whole-lung-airway model. Part II: Dry powder inhaler application.Comput Biol Med. 2017 May 1;84:247-253. doi: 10.1016/j.compbiomed.2016.10.025. Epub 2016 Nov 3. Comput Biol Med. 2017. PMID: 27836120
-
A dry powder inhaler with reduced mouth-throat deposition.J Aerosol Med. 2006 Summer;19(2):168-74. doi: 10.1089/jam.2006.19.168. J Aerosol Med. 2006. PMID: 16796541
-
Supercritical Fluid Particle Design of DPI Formulations (Review).Curr Pharm Des. 2015;21(19):2516-42. doi: 10.2174/1381612821666150416100201. Curr Pharm Des. 2015. PMID: 25876911 Review.
-
Recent advances in capsule-based dry powder inhaler technology.Multidiscip Respir Med. 2017 May 22;12:11. doi: 10.1186/s40248-017-0092-5. eCollection 2017. Multidiscip Respir Med. 2017. PMID: 28536654 Free PMC article. Review.
Cited by
-
Dry Powder Inhalers: A Focus on Advancements in Novel Drug Delivery Systems.J Drug Deliv. 2016;2016:8290963. doi: 10.1155/2016/8290963. Epub 2016 Oct 27. J Drug Deliv. 2016. PMID: 27867663 Free PMC article. Review.
-
Inhalable Nano-Dimpled Microspheres Containing Budesonide-PLGA for Improved Aerodynamic Performance.Int J Nanomedicine. 2022 Aug 3;17:3405-3419. doi: 10.2147/IJN.S372582. eCollection 2022. Int J Nanomedicine. 2022. PMID: 35945926 Free PMC article.
References
-
- Naikwade S. R., Bajaj A. N., Gurav P., Gatne M. M., Singh Soni P. Development of budesonide microparticles using spray-drying technology for pulmonary administration: design, characterization, in vitro evaluation, and in vivo efficacy study. AAPS PharmSciTech. 2009;10(3):993–1012. doi: 10.1208/s12249-009-9290-6. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

