14-3-3 Proteins regulate mutant LRRK2 kinase activity and neurite shortening
- PMID: 26546614
- PMCID: PMC4690493
- DOI: 10.1093/hmg/ddv453
14-3-3 Proteins regulate mutant LRRK2 kinase activity and neurite shortening
Abstract
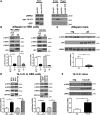
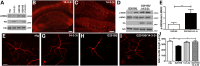
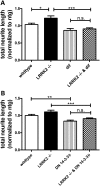
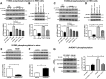
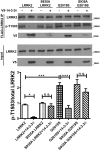
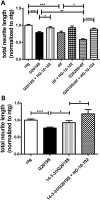
Mutations in leucine-rich repeat kinase 2 (LRRK2) are the most common known cause of inherited Parkinson's disease (PD), and LRRK2 is a risk factor for idiopathic PD. How LRRK2 function is regulated is not well understood. Recently, the highly conserved 14-3-3 proteins, which play a key role in many cellular functions including cell death, have been shown to interact with LRRK2. In this study, we investigated whether 14-3-3s can regulate mutant LRRK2-induced neurite shortening and kinase activity. In the presence of 14-3-3θ overexpression, neurite length of primary neurons from BAC transgenic G2019S-LRRK2 mice returned back to wild-type levels. Similarly, 14-3-3θ overexpression reversed neurite shortening in neuronal cultures from BAC transgenic R1441G-LRRK2 mice. Conversely, inhibition of 14-3-3s by the pan-14-3-3 inhibitor difopein or dominant-negative 14-3-3θ further reduced neurite length in G2019S-LRRK2 cultures. Since G2019S-LRRK2 toxicity is likely mediated through increased kinase activity, we examined 14-3-3θ's effects on LRRK2 kinase activity. 14-3-3θ overexpression reduced the kinase activity of G2019S-LRRK2, while difopein promoted the kinase activity of G2019S-LRRK2. The ability of 14-3-3θ to reduce LRRK2 kinase activity required direct binding of 14-3-3θ with LRRK2. The potentiation of neurite shortening by difopein in G2019S-LRRK2 neurons was reversed by LRRK2 kinase inhibitors. Taken together, we conclude that 14-3-3θ can regulate LRRK2 and reduce the toxicity of mutant LRRK2 through a reduction of kinase activity.
© The Author 2015. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures



Similar articles
-
14-3-3 phosphorylation inhibits 14-3-3θ's ability to regulate LRRK2 kinase activity and toxicity.Hum Mol Genet. 2024 Nov 20;33(23):2071-2083. doi: 10.1093/hmg/ddae142. Hum Mol Genet. 2024. PMID: 39324210
-
14-3-3 phosphorylation inhibits 14-3-3θ's ability to regulate LRRK2 kinase activity.bioRxiv [Preprint]. 2023 May 30:2023.05.27.542591. doi: 10.1101/2023.05.27.542591. bioRxiv. 2023. Update in: Hum Mol Genet. 2024 Nov 20;33(23):2071-2083. doi: 10.1093/hmg/ddae142 PMID: 37398189 Free PMC article. Updated. Preprint.
-
Inhibition of excessive mitochondrial fission reduced aberrant autophagy and neuronal damage caused by LRRK2 G2019S mutation.Hum Mol Genet. 2013 Nov 15;22(22):4545-61. doi: 10.1093/hmg/ddt301. Epub 2013 Jun 27. Hum Mol Genet. 2013. PMID: 23813973
-
Leucine-rich repeat kinase 2 inhibitors: a review of recent patents (2011 - 2013).Expert Opin Ther Pat. 2014 Jul;24(7):745-57. doi: 10.1517/13543776.2014.907275. Epub 2014 Jun 11. Expert Opin Ther Pat. 2014. PMID: 24918198 Review.
-
α-Synuclein, leucine-rich repeat kinase-2, and manganese in the pathogenesis of Parkinson disease.Neurotoxicology. 2011 Oct;32(5):622-9. doi: 10.1016/j.neuro.2011.01.003. Epub 2011 Jan 14. Neurotoxicology. 2011. PMID: 21238487 Free PMC article. Review.
Cited by
-
Chronic stress dysregulates the Hippo/YAP/14-3-3η pathway and induces mitochondrial damage in basolateral amygdala in a mouse model of depression.Theranostics. 2024 Jun 11;14(9):3653-3673. doi: 10.7150/thno.92676. eCollection 2024. Theranostics. 2024. PMID: 38948066 Free PMC article.
-
Binding of the Human 14-3-3 Isoforms to Distinct Sites in the Leucine-Rich Repeat Kinase 2.Front Neurosci. 2020 Apr 7;14:302. doi: 10.3389/fnins.2020.00302. eCollection 2020. Front Neurosci. 2020. PMID: 32317922 Free PMC article.
-
How Parkinson's Disease-Linked LRRK2 Mutations Affect Different CNS Cell Types.J Parkinsons Dis. 2024;14(7):1331-1352. doi: 10.3233/JPD-230432. J Parkinsons Dis. 2024. PMID: 38905056 Free PMC article. Review.
-
Pharmacodynamic Biomarkers for Emerging LRRK2 Therapeutics.Front Neurosci. 2020 Aug 6;14:807. doi: 10.3389/fnins.2020.00807. eCollection 2020. Front Neurosci. 2020. PMID: 32903744 Free PMC article. Review.
-
Pathogenic LRRK2 requires secondary factors to induce cellular toxicity.Biosci Rep. 2020 Oct 30;40(10):BSR20202225. doi: 10.1042/BSR20202225. Biosci Rep. 2020. PMID: 32975566 Free PMC article.
References
-
- Macleod A.D., Taylor K.S., Counsell C.E. (2014) Mortality in Parkinson's disease: a systematic review and meta-analysis. Mov. Disord., 29, 1615–1622. - PubMed
-
- Paisan-Ruiz C., Jain S., Evans E.W., Gilks W.P., Simon J., van der Brug M., Lopez de Munain A., Aparicio S., Gil A.M., Khan N. et al. (2004) Cloning of the gene containing mutations that cause PARK8-linked Parkinson's disease. Neuron, 44, 595–600. - PubMed
-
- Zimprich A., Biskup S., Leitner P., Lichtner P., Farrer M., Lincoln S., Kachergus J., Hulihan M., Uitti R.J., Calne D.B. et al. (2004) Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron, 44, 601–607. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

