Regulation of inflammatory biomarkers by intravenous methylprednisolone in pediatric ARDS patients: Results from a double-blind, placebo-controlled randomized pilot trial
- PMID: 26545141
- PMCID: PMC4666843
- DOI: 10.1016/j.cyto.2015.10.007
Regulation of inflammatory biomarkers by intravenous methylprednisolone in pediatric ARDS patients: Results from a double-blind, placebo-controlled randomized pilot trial
Abstract
Objective: A double-blind, randomized controlled trial showed that low-dose glucocorticoid therapy in pediatric ARDS patients is feasible and may improve both ventilation and oxygenation indices in these patients. However, the molecular mechanisms underlying potential changes in outcomes remain unclear. Based on these clinical findings, this study was designed to examine the effects of intravenous methylprednisolone on circulating inflammatory biomarkers in pediatric ARDS patients.
Design: Double-blind, placebo-controlled randomized trial with blood collection on study entry and day 7.
Setting: Tertiary care children's hospital.
Patients: Children (0-18years) with ARDS undergoing mechanical ventilation.
Interventions: 35 children were randomized within 72h of mechanical ventilation. The glucocorticoid group received methylprednisolone 2mg/kg loading dose followed by 1mg/kg/day continuous infusion from days 1 to 7. Both groups were ventilated following the ARDSnet recommendations. WBC and differential cell counts, plasma cytokines and CRP levels, and coagulation parameters were analyzed on days 0 and 7.
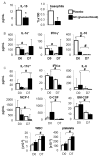
Results: At study entry, the placebo group had higher IL-15 and basophil levels. On day 7, in comparison to study entry, the placebo group had lower IL-1α, IFN-γ and IL-10 levels. The glucocorticoid group had lower INF-α, IL-6, IL-10, MCP-1, G-CSF and GM-CSF levels, and higher IL-17α levels on day 7 in comparison to study entry. Total and differential cell counts remained unchanged within the placebo group between days 0 and 7, whereas in the glucocorticoid group total WBC and platelets counts were increased on day 7. Pearson's correlation studies within the placebo and glucocorticoid groups revealed positive and negative correlations between cytokine levels, cell counts, coagulation parameters and relevant clinical parameters of disease severity identified in our previous study. Multiple regression models identified several cytokines as predictors for alterations in clinical parameters of disease severity.
Conclusion: This pilot study shows the feasibility of simultaneously measuring multiple inflammatory cytokines, cell counts and coagulation parameters in pediatric ARDS patients. We report statistical models that may be useful for future, larger trials to predict ARDS severity and outcomes.
Trial registration: ClinicalTrials.gov NCT01274260.
Keywords: ARDS; Chemokines; Cytokines; Glucocorticoids; Inflammation; Lung injury; Mediators; Pediatrics; Steroids.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
Plasma Biomarker Analysis in Pediatric ARDS: Generating Future Framework from a Pilot Randomized Control Trial of Methylprednisolone: A Framework for Identifying Plasma Biomarkers Related to Clinical Outcomes in Pediatric ARDS.Front Pediatr. 2016 Mar 31;4:31. doi: 10.3389/fped.2016.00031. eCollection 2016. Front Pediatr. 2016. PMID: 27066464 Free PMC article.
-
Double-blind, placebo-controlled pilot randomized trial of methylprednisolone infusion in pediatric acute respiratory distress syndrome.Pediatr Crit Care Med. 2015 Mar;16(3):e74-81. doi: 10.1097/PCC.0000000000000349. Pediatr Crit Care Med. 2015. PMID: 25634565 Clinical Trial.
-
Treatment of COVID-19 pneumonia with glucocorticoids (CORTIVID): a structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Jan 11;22(1):43. doi: 10.1186/s13063-020-04999-4. Trials. 2021. PMID: 33430891 Free PMC article.
-
Prolonged glucocorticoid treatment is associated with improved ARDS outcomes: analysis of individual patients' data from four randomized trials and trial-level meta-analysis of the updated literature.Intensive Care Med. 2016 May;42(5):829-840. doi: 10.1007/s00134-015-4095-4. Epub 2015 Oct 27. Intensive Care Med. 2016. PMID: 26508525 Review.
-
Glucocorticoids and acute lung injury.Crit Care Med. 2003 Apr;31(4 Suppl):S253-7. doi: 10.1097/01.CCM.0000057900.19201.55. Crit Care Med. 2003. PMID: 12682449 Review.
Cited by
-
PARP-1 Inhibition Repressed Imbalance of Th17 and Treg Cells in Preterm Rats with Intrauterine Infection-Induced Acute Respiratory Distress Syndrome by Reducing the Expression Level of IL-6.J Healthc Eng. 2022 Feb 12;2022:1255674. doi: 10.1155/2022/1255674. eCollection 2022. J Healthc Eng. 2022. PMID: 35190759 Free PMC article.
-
Effect of a single high dose of vitamin D3 on cytokines, chemokines, and growth factor in patients with moderate to severe COVID-19.Am J Clin Nutr. 2022 Mar 4;115(3):790-798. doi: 10.1093/ajcn/nqab426. Am J Clin Nutr. 2022. PMID: 35020796 Free PMC article. Clinical Trial.
-
Plasma Biomarker Analysis in Pediatric ARDS: Generating Future Framework from a Pilot Randomized Control Trial of Methylprednisolone: A Framework for Identifying Plasma Biomarkers Related to Clinical Outcomes in Pediatric ARDS.Front Pediatr. 2016 Mar 31;4:31. doi: 10.3389/fped.2016.00031. eCollection 2016. Front Pediatr. 2016. PMID: 27066464 Free PMC article.
-
Biomarkers in Pediatric ARDS: Future Directions.Front Pediatr. 2016 Jun 1;4:55. doi: 10.3389/fped.2016.00055. eCollection 2016. Front Pediatr. 2016. PMID: 27313995 Free PMC article. Review.
-
Corticosteroids in Acute Lung Injury: The Dilemma Continues.Int J Mol Sci. 2019 Sep 25;20(19):4765. doi: 10.3390/ijms20194765. Int J Mol Sci. 2019. PMID: 31557974 Free PMC article. Review.
References
-
- Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet. 1967;2(7511):319–323. - PubMed
-
- Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. American journal of respiratory and critical care medicine. 1994;149(3 Pt 1):818–824. - PubMed
-
- Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. The New England journal of medicine. 2005;353(16):1685–1693. - PubMed
-
- Zimmerman JJ, Akhtar SR, Caldwell E, Rubenfeld GD. Incidence and outcomes of pediatric acute lung injury. Pediatrics. 2009;124(1):87–95. - PubMed
-
- Zambon M, Vincent JL. Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest. 2008;133(5):1120–1127. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

