Breaking down the COP9 Signalsome in the heart: how inactivating a protein ubiquitin ligase increases protein ubiquitylation and protects the heart
- PMID: 26541679
- PMCID: PMC4686129
- DOI: 10.1161/CIRCRESAHA.115.307644
Breaking down the COP9 Signalsome in the heart: how inactivating a protein ubiquitin ligase increases protein ubiquitylation and protects the heart
Keywords: Editorials; proteasome endopeptidase complex; proteolysis; ubiquitination.
Figures
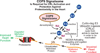
Comment on
-
COP9 signalosome controls the degradation of cytosolic misfolded proteins and protects against cardiac proteotoxicity.Circ Res. 2015 Nov 6;117(11):956-66. doi: 10.1161/CIRCRESAHA.115.306783. Epub 2015 Sep 17. Circ Res. 2015. PMID: 26383969 Free PMC article.
Similar articles
-
COP9 signalosome controls the degradation of cytosolic misfolded proteins and protects against cardiac proteotoxicity.Circ Res. 2015 Nov 6;117(11):956-66. doi: 10.1161/CIRCRESAHA.115.306783. Epub 2015 Sep 17. Circ Res. 2015. PMID: 26383969 Free PMC article.
-
Search and destroy: the role of protein quality control in maintaining cardiac function.J Mol Cell Cardiol. 2006 Apr;40(4):438-41. doi: 10.1016/j.yjmcc.2006.01.003. Epub 2006 Mar 6. J Mol Cell Cardiol. 2006. PMID: 16516914 No abstract available.
-
Inhibition of Mutant αB Crystallin-Induced Protein Aggregation by a Molecular Tweezer.J Am Heart Assoc. 2017 Aug 8;6(8):e006182. doi: 10.1161/JAHA.117.006182. J Am Heart Assoc. 2017. PMID: 28862927 Free PMC article.
-
Mitochondrial Ubiquitin Ligase in Cardiovascular Disorders.Adv Exp Med Biol. 2017;982:327-333. doi: 10.1007/978-3-319-55330-6_17. Adv Exp Med Biol. 2017. PMID: 28551795 Review.
-
Large-scale characterization of the murine cardiac proteome.Methods Mol Biol. 2013;1005:1-10. doi: 10.1007/978-1-62703-386-2_1. Methods Mol Biol. 2013. PMID: 23606244 Review.
Cited by
-
Induction of Proteasome Subunit Low Molecular Weight Protein (LMP)-2 Is Required to Induce Active Remodeling in Adult Rat Ventricular Cardiomyocytes.Med Sci (Basel). 2020 May 1;8(2):21. doi: 10.3390/medsci8020021. Med Sci (Basel). 2020. PMID: 32370048 Free PMC article.
References
-
- Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. - PubMed
-
- Christians ES, Mustafi SB, Benjamin IJ. Chaperones and cardiac misfolding protein diseases. Curr Protein Pept Sci. 2014;15:189–204. - PubMed
-
- Kriegenburg F, Ellgaard L, Hartmann-Petersen R. Molecular chaperones in targeting misfolded proteins for ubiquitin-dependent degradation. FEBS J. 2012;279:532–542. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

