Genome-wide endogenous DAF-16/FOXO recruitment dynamics during lowered insulin signalling in C. elegans
- PMID: 26539642
- PMCID: PMC4747164
- DOI: 10.18632/oncotarget.6282
Genome-wide endogenous DAF-16/FOXO recruitment dynamics during lowered insulin signalling in C. elegans
Abstract
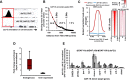
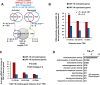
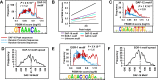
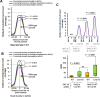
Lowering insulin-IGF-1-like signalling (IIS) activates FOXO transcription factors (TF) to extend life span across species. To study the dynamics of FOXO chromatin occupancy under this condition in C. elegans, we report the first recruitment profile of endogenous DAF-16 and show that the response is conserved. DAF-16 predominantly acts as a transcriptional activator and binding within the 0.5 kb promoter-proximal region results in maximum induction of downstream targets that code for proteins involved in detoxification and longevity. Interestingly, genes that are activated under low IIS already have higher DAF-16 recruited to their promoters in WT. DAF-16 binds to variants of the FOXO consensus sequence in the promoter proximal regions of genes that are exclusively targeted during low IIS. We also define a set of 'core' direct targets, after comparing multiple studies, which tend to co-express and contribute robustly towards IIS-associated phenotypes. Additionally, we show that nuclear hormone receptor DAF-12 as well as zinc-finger TF EOR-1 may bind DNA in close proximity to DAF-16 and distinct TF classes that are direct targets of DAF-16 may be instrumental in regulating its indirect targets. Together, our study provides fundamental insights into the transcriptional biology of FOXO/DAF-16 and gene regulation downstream of the IIS pathway.
Keywords: C. elegans; ChIP-seq; DAF-16; FOXO; Gerotarget; transcription.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
The C. elegans adult neuronal IIS/FOXO transcriptome reveals adult phenotype regulators.Nature. 2016 Jan 7;529(7584):92-6. doi: 10.1038/nature16483. Epub 2015 Dec 14. Nature. 2016. PMID: 26675724 Free PMC article.
-
DAF-16 target genes that control C. elegans life-span and metabolism.Science. 2003 Apr 25;300(5619):644-7. doi: 10.1126/science.1083614. Epub 2003 Apr 10. Science. 2003. PMID: 12690206
-
Towards understanding the lifespan extension by reduced insulin signaling: bioinformatics analysis of DAF-16/FOXO direct targets in Caenorhabditis elegans.Oncotarget. 2016 Apr 12;7(15):19185-92. doi: 10.18632/oncotarget.8313. Oncotarget. 2016. PMID: 27027346 Free PMC article.
-
The search for DAF-16/FOXO transcriptional targets: approaches and discoveries.Exp Gerontol. 2006 Oct;41(10):910-21. doi: 10.1016/j.exger.2006.06.040. Epub 2006 Aug 24. Exp Gerontol. 2006. PMID: 16934425 Review.
-
DAF-16: FOXO in the Context of C. elegans.Curr Top Dev Biol. 2018;127:1-21. doi: 10.1016/bs.ctdb.2017.11.007. Epub 2018 Feb 2. Curr Top Dev Biol. 2018. PMID: 29433733 Review.
Cited by
-
Combinatorial transcriptomic and genetic dissection of insulin/IGF-1 signaling-regulated longevity in Caenorhabditis elegans.Aging Cell. 2024 Jul;23(7):e14151. doi: 10.1111/acel.14151. Epub 2024 Mar 26. Aging Cell. 2024. PMID: 38529797 Free PMC article.
-
In vivo neuroprotective capacity of a Dunaliella salina extract - comprehensive transcriptomics and metabolomics study.NPJ Sci Food. 2024 Jan 10;8(1):4. doi: 10.1038/s41538-023-00246-7. NPJ Sci Food. 2024. PMID: 38200022 Free PMC article.
-
MiR-150 promotes cellular metastasis in non-small cell lung cancer by targeting FOXO4.Sci Rep. 2016 Dec 15;6:39001. doi: 10.1038/srep39001. Sci Rep. 2016. PMID: 27976702 Free PMC article.
-
Long Noncoding RNA XR007793 Regulates Proliferation and Migration of Vascular Smooth Muscle Cell via Suppressing miR-23b.Med Sci Monit. 2018 Aug 24;24:5895-5903. doi: 10.12659/MSM.908902. Med Sci Monit. 2018. PMID: 30141428 Free PMC article.
-
Temporal transitions in the post-mitotic nervous system of Caenorhabditis elegans.Nature. 2021 Dec;600(7887):93-99. doi: 10.1038/s41586-021-04071-4. Epub 2021 Nov 10. Nature. 2021. PMID: 34759317 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

