Cell-Permeable Peptide Targeting the Nrf2-Keap1 Interaction: A Potential Novel Therapy for Global Cerebral Ischemia
- PMID: 26538645
- PMCID: PMC4635127
- DOI: 10.1523/JNEUROSCI.1304-15.2015
Cell-Permeable Peptide Targeting the Nrf2-Keap1 Interaction: A Potential Novel Therapy for Global Cerebral Ischemia
Abstract
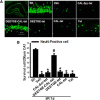
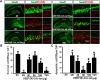
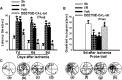
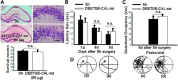
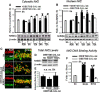
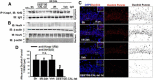
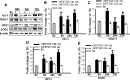
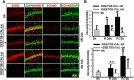
The current study examined efficacy of a small Tat (trans-activator of transcription)-conjugated peptide activator of the Nrf2 (nuclear factor-E2-related factor-2) antioxidant/cell-defense pathway as a potential injury-specific, novel neuroprotectant against global cerebral ischemia (GCI). A competitive peptide, DEETGE-CAL-Tat, was designed to facilitate Nrf2 activation by disrupting interaction of Nrf2 with Keap1 (kelch-like ECH-associated protein 1), a protein that sequesters Nrf2 in the cytoplasm and thereby inactivates it. The DEETGE-CAL-Tat peptide contained the critical sequence DEETGE for the Nrf2-Keap1 interaction, the cell transduction domain of the HIV-Tat protein, and the cleavage sequence of calpain, which is sensitive to Ca(2+) increase and allows injury-specific activation of Nrf2. Using an animal model of GCI, we demonstrated that pretreatment with the DEETGE-CAL-Tat peptide markedly decreased Nrf2 interaction with Keap1 in the rat hippocampal CA1 region after GCI, and enhanced Nrf2 nuclear translocation and DNA binding. The DEETGE-CAL-Tat peptide also induced Nrf2 antioxidant/cytoprotective target genes, reduced oxidative stress, and induced strong neuroprotection and marked preservation of hippocampal-dependent cognitive function after GCI. These effects were specific as control peptides lacked neuroprotective ability. Intriguingly, the DEETGE-CAL-Tat peptide effects were also injury specific, as it had no effect upon neuronal survival or cognitive performance in sham nonischemic animals. Of significant interest, peripheral, postischemia administration of the DEETGE-CAL-Tat peptide from days 1-9 after GCI also induced robust neuroprotection and strongly preserved hippocampal-dependent cognitive function. Based on its robust neuroprotective and cognitive-preserving effects, and its unique injury-specific activation properties, the DEETGE-CAL-Tat peptide represents a novel, and potentially promising new therapeutic modality for the treatment of GCI.
Significance statement: The current study demonstrates that DEETGE-CAL-Tat, a novel peptide activator of a key antioxidant gene transcription pathway in the hippocampus after global cerebral ischemia, can exert robust neuroprotection and preservation of cognitive function. A unique feature of the peptide is that its beneficial effects are injury specific. This feature is attractive as it targets drug activation specifically in the site of injury, and likely would lead to a reduction of undesirable side effects if translatable to the clinic. Due to its injury-specific activation, robust neuroprotection, and cognitive-preserving effects, this novel peptide may represent a much-needed therapeutic advance that could have efficacy in the treatment of global cerebral ischemia.
Keywords: cardiac arrest; cognitive defect; hippocampus CA1 region; neuroprotection; peptide; reactive oxidative species.
Copyright © 2015 the authors 0270-6474/15/3514728-13$15.00/0.
Figures
Similar articles
-
A novel strategy to activate cytoprotective genes in the injured brain.Biochem Biophys Res Commun. 2011 Apr 15;407(3):501-6. doi: 10.1016/j.bbrc.2011.03.046. Epub 2011 Mar 22. Biochem Biophys Res Commun. 2011. PMID: 21414291 Free PMC article.
-
[Keap1-tat peptide attenuates oxidative stress damage in hippocampal CA1 region and learning and memory deficits following global cerebral ischemia].Beijing Da Xue Xue Bao Yi Xue Ban. 2016 Feb 18;48(1):154-9. Beijing Da Xue Xue Bao Yi Xue Ban. 2016. PMID: 26885927 Chinese.
-
Genistein attenuates ischemic oxidative damage and behavioral deficits via eNOS/Nrf2/HO-1 signaling.Hippocampus. 2013 Jul;23(7):634-47. doi: 10.1002/hipo.22126. Epub 2013 Jun 3. Hippocampus. 2013. PMID: 23536494
-
Small molecule modulators of Keap1-Nrf2-ARE pathway as potential preventive and therapeutic agents.Med Res Rev. 2012 Jul;32(4):687-726. doi: 10.1002/med.21257. Epub 2012 May 1. Med Res Rev. 2012. PMID: 22549716 Free PMC article. Review.
-
The Keap1-Nrf2 cell defense pathway--a promising therapeutic target?Adv Pharmacol. 2012;63:43-79. doi: 10.1016/B978-0-12-398339-8.00002-1. Adv Pharmacol. 2012. PMID: 22776639 Review.
Cited by
-
Differential roles of exogenous protein disulfide isomerase A3 on proliferating cell and neuroblast numbers in the normal and ischemic gerbils.Brain Behav. 2020 Mar;10(3):e01534. doi: 10.1002/brb3.1534. Epub 2020 Jan 20. Brain Behav. 2020. PMID: 31957985 Free PMC article.
-
Relationship between oxidative stress and nuclear factor-erythroid-2-related factor 2 signaling in diabetic cardiomyopathy (Review).Exp Ther Med. 2021 Jul;22(1):678. doi: 10.3892/etm.2021.10110. Epub 2021 Apr 25. Exp Ther Med. 2021. PMID: 33986843 Free PMC article. Review.
-
PTD4 Peptide Increases Neural Viability in an In Vitro Model of Acute Ischemic Stroke.Int J Mol Sci. 2021 Jun 4;22(11):6086. doi: 10.3390/ijms22116086. Int J Mol Sci. 2021. PMID: 34200045 Free PMC article.
-
Repurposing dimethyl fumarate as an antiepileptogenic and disease-modifying treatment for drug-resistant epilepsy.J Transl Med. 2023 Nov 8;21(1):796. doi: 10.1186/s12967-023-04695-2. J Transl Med. 2023. PMID: 37940957 Free PMC article.
-
G-protein-coupled estrogen receptor activation upregulates interleukin-1 receptor antagonist in the hippocampus after global cerebral ischemia: implications for neuronal self-defense.J Neuroinflammation. 2020 Feb 1;17(1):45. doi: 10.1186/s12974-020-1715-x. J Neuroinflammation. 2020. PMID: 32007102 Free PMC article.
References
-
- Brillman J. Central nervous system complications in coronary artery bypass graft surgery. Neurol Clin. 1993;11:475–495. - PubMed
-
- Chen HH, Chen YT, Huang YW, Tsai HJ, Kuo CC. 4-Ketopinoresinol, a novel naturally occurring ARE activator, induces the Nrf2/HO-1 axis and protects against oxidative stress-induced cell injury via activation of PI3K/AKT signaling. Free Radic Biol Med. 2012;52:1054–1066. doi: 10.1016/j.freeradbiomed.2011.12.012. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
