2-Hydroxypropyl-β-Cyclodextrin Acts as a Novel Anticancer Agent
- PMID: 26535909
- PMCID: PMC4633159
- DOI: 10.1371/journal.pone.0141946
2-Hydroxypropyl-β-Cyclodextrin Acts as a Novel Anticancer Agent
Abstract
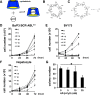
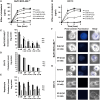
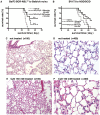
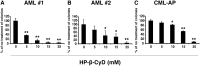
2-Hydroxypropyl-β-cyclodextrin (HP-β-CyD) is a cyclic oligosaccharide that is widely used as an enabling excipient in pharmaceutical formulations, but also as a cholesterol modifier. HP-β-CyD has recently been approved for the treatment of Niemann-Pick Type C disease, a lysosomal lipid storage disorder, and is used in clinical practice. Since cholesterol accumulation and/or dysregulated cholesterol metabolism has been described in various malignancies, including leukemia, we hypothesized that HP-β-CyD itself might have anticancer effects. This study provides evidence that HP-β-CyD inhibits leukemic cell proliferation at physiologically available doses. First, we identified the potency of HP-β-CyD in vitro against various leukemic cell lines derived from acute myeloid leukemia (AML), acute lymphoblastic leukemia and chronic myeloid leukemia (CML). HP-β-CyD treatment reduced intracellular cholesterol resulting in significant leukemic cell growth inhibition through G2/M cell-cycle arrest and apoptosis. Intraperitoneal injection of HP-β-CyD significantly improved survival in leukemia mouse models. Importantly, HP-β-CyD also showed anticancer effects against CML cells expressing a T315I BCR-ABL mutation (that confers resistance to most ABL tyrosine kinase inhibitors), and hypoxia-adapted CML cells that have characteristics of leukemic stem cells. In addition, colony forming ability of human primary AML and CML cells was inhibited by HP-β-CyD. Systemic administration of HP-β-CyD to mice had no significant adverse effects. These data suggest that HP-β-CyD is a promising anticancer agent regardless of disease or cellular characteristics.
Conflict of interest statement
Figures



Similar articles
-
Folate-Appended Hydroxypropyl-β-Cyclodextrin Induces Autophagic Cell Death in Acute Myeloid Leukemia Cells.Int J Mol Sci. 2023 Nov 24;24(23):16720. doi: 10.3390/ijms242316720. Int J Mol Sci. 2023. PMID: 38069042 Free PMC article.
-
Triptolide inhibits Bcr-Abl transcription and induces apoptosis in STI571-resistant chronic myelogenous leukemia cells harboring T315I mutation.Clin Cancer Res. 2009 Mar 1;15(5):1686-97. doi: 10.1158/1078-0432.CCR-08-2141. Epub 2009 Feb 24. Clin Cancer Res. 2009. PMID: 19240172
-
HS-438, a new inhibitor of imatinib-resistant BCR-ABL T315I mutation in chronic myeloid leukemia.Cancer Lett. 2014 Jun 28;348(1-2):50-60. doi: 10.1016/j.canlet.2014.03.012. Epub 2014 Mar 18. Cancer Lett. 2014. PMID: 24657654
-
Mechanisms of resistance to BCR-ABL TKIs and the therapeutic strategies: A review.Crit Rev Oncol Hematol. 2015 Mar;93(3):277-92. doi: 10.1016/j.critrevonc.2014.11.001. Epub 2014 Nov 13. Crit Rev Oncol Hematol. 2015. PMID: 25500000 Review.
-
Early experience with compassionate use of 2 hydroxypropyl-beta-cyclodextrin for Niemann-Pick type C disease: review of initial published cases.Neurol Sci. 2017 May;38(5):727-743. doi: 10.1007/s10072-017-2833-9. Epub 2017 Feb 2. Neurol Sci. 2017. PMID: 28155026 Review.
Cited by
-
The RORɣ/SREBP2 pathway is a master regulator of cholesterol metabolism and serves as potential therapeutic target in t(4;11) leukemia.Oncogene. 2024 Jan;43(4):281-293. doi: 10.1038/s41388-023-02903-3. Epub 2023 Nov 29. Oncogene. 2024. PMID: 38030791 Free PMC article.
-
Synthetic Receptors for Early Detection and Treatment of Cancer.Biosensors (Basel). 2023 Oct 25;13(11):953. doi: 10.3390/bios13110953. Biosensors (Basel). 2023. PMID: 37998127 Free PMC article. Review.
-
Gold nanoparticles stabilized with βcyclodextrin-2-amino-4-(4-chlorophenyl)thiazole complex: A novel system for drug transport.PLoS One. 2017 Oct 11;12(10):e0185652. doi: 10.1371/journal.pone.0185652. eCollection 2017. PLoS One. 2017. PMID: 29020065 Free PMC article.
-
Folate-Appended Hydroxypropyl-β-Cyclodextrin Induces Autophagic Cell Death in Acute Myeloid Leukemia Cells.Int J Mol Sci. 2023 Nov 24;24(23):16720. doi: 10.3390/ijms242316720. Int J Mol Sci. 2023. PMID: 38069042 Free PMC article.
-
Hydroxypropyl-β-Cyclodextrin Depletes Membrane Cholesterol and Inhibits SARS-CoV-2 Entry into HEK293T-ACEhi Cells.Pathogens. 2023 Apr 27;12(5):647. doi: 10.3390/pathogens12050647. Pathogens. 2023. PMID: 37242317 Free PMC article.
References
-
- Wakeling AE, Guy SP, Woodburn JR, Ashton SE, Curry BJ, Barker AJ, et al. ZD1839 (Iressa): an orally active inhibitor of epidermal growth factor signaling with potential for cancer therapy. Cancer Res. 2002;62: 5749–5754. - PubMed
-
- Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344: 1031–1037. - PubMed
-
- Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343: 425–430. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

