Relating Chemical Structure to Cellular Response: An Integrative Analysis of Gene Expression, Bioactivity, and Structural Data Across 11,000 Compounds
- PMID: 26535158
- PMCID: PMC4625862
- DOI: 10.1002/psp4.12009
Relating Chemical Structure to Cellular Response: An Integrative Analysis of Gene Expression, Bioactivity, and Structural Data Across 11,000 Compounds
Abstract
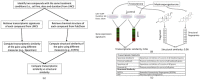
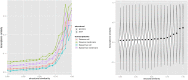
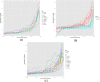
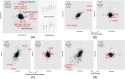
A central premise in systems pharmacology is that structurally similar compounds have similar cellular responses; however, this principle often does not hold. One of the most widely used measures of cellular response is gene expression. By integrating gene expression data from Library of Integrated Network-based Cellular Signatures (LINCS) with chemical structure and bioactivity data from PubChem, we performed a large-scale correlation analysis of chemical structures and gene expression profiles of over 11,000 compounds taking into account confounding factors such as biological conditions (e.g., cell line, dose) and bioactivities. We found that structurally similar compounds do indeed yield similar gene expression profiles. There is an ∼20% chance that two structurally similar compounds (Tanimoto Coefficient ≥ 0.85) share significantly similar gene expression profiles. Regardless of structural similarity, two compounds tend to share similar gene expression profiles in a cell line when they are administrated at a higher dose or when the cell line is sensitive to both compounds.
Figures

Similar articles
-
Molecular Structure-Based Large-Scale Prediction of Chemical-Induced Gene Expression Changes.J Chem Inf Model. 2017 Sep 25;57(9):2194-2202. doi: 10.1021/acs.jcim.7b00281. Epub 2017 Aug 22. J Chem Inf Model. 2017. PMID: 28796500
-
Large-scale integration of small molecule-induced genome-wide transcriptional responses, Kinome-wide binding affinities and cell-growth inhibition profiles reveal global trends characterizing systems-level drug action.Front Genet. 2014 Sep 30;5:342. doi: 10.3389/fgene.2014.00342. eCollection 2014. Front Genet. 2014. PMID: 25324859 Free PMC article.
-
Public Domain HTS Fingerprints: Design and Evaluation of Compound Bioactivity Profiles from PubChem's Bioassay Repository.J Chem Inf Model. 2016 Feb 22;56(2):390-8. doi: 10.1021/acs.jcim.5b00498. Epub 2016 Jan 14. J Chem Inf Model. 2016. PMID: 26898267
-
Systems analysis of protein modification and cellular responses induced by electrophile stress.Acc Chem Res. 2010 May 18;43(5):673-83. doi: 10.1021/ar900286y. Acc Chem Res. 2010. PMID: 20218676 Free PMC article. Review.
-
Linking tumor cell cytotoxicity to mechanism of drug action: an integrated analysis of gene expression, small-molecule screening and structural databases.Proteins. 2005 May 15;59(3):403-33. doi: 10.1002/prot.20392. Proteins. 2005. PMID: 15778971 Review.
Cited by
-
GraphDTI: A robust deep learning predictor of drug-target interactions from multiple heterogeneous data.J Cheminform. 2021 Aug 11;13(1):58. doi: 10.1186/s13321-021-00540-0. J Cheminform. 2021. PMID: 34380569 Free PMC article.
-
From drug repositioning to target repositioning: prediction of therapeutic targets using genetically perturbed transcriptomic signatures.Bioinformatics. 2022 Jun 24;38(Suppl 1):i68-i76. doi: 10.1093/bioinformatics/btac240. Bioinformatics. 2022. PMID: 35758779 Free PMC article.
-
Predicting drug-induced transcriptome responses of a wide range of human cell lines by a novel tensor-train decomposition algorithm.Bioinformatics. 2019 Jul 15;35(14):i191-i199. doi: 10.1093/bioinformatics/btz313. Bioinformatics. 2019. PMID: 31510663 Free PMC article.
-
Recent Advances in Cancer Drug Discovery Through the Use of Phenotypic Reporter Systems, Connectivity Mapping, and Pooled CRISPR Screening.Front Pharmacol. 2022 Jun 20;13:852143. doi: 10.3389/fphar.2022.852143. eCollection 2022. Front Pharmacol. 2022. PMID: 35795568 Free PMC article. Review.
-
Combined inhibition of atypical PKC and histone deacetylase 1 is cooperative in basal cell carcinoma treatment.JCI Insight. 2017 Nov 2;2(21):e97071. doi: 10.1172/jci.insight.97071. JCI Insight. 2017. PMID: 29093271 Free PMC article.
References
-
- Martin YC, Kofron JL. Traphagen LM. Do structurally similar molecules have similar biological activity? J. Med. Chem. 2002;45:4350–4358. & ) - PubMed
-
- Stumpfe D. Bajorath J. Exploring activity cliffs in medicinal chemistry. J. Med. Chem. 2012;55:2932–2942. & ) - PubMed
-
- Bender A. Glen RC. Molecular similarity: a key technique in molecular informatics. Organ. Biomol. Chem. 2004;2:3204–3218. & ) - PubMed
-
- Nissen SE. Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N. Engl. J. Med. 2007;356:2457–2471. & ) - PubMed
-
- Menon KVN, Angulo P. Lindor KD. Severe cholestatic hepatitis from troglitazone in a patient with nonalcoholic steatohepatitis and diabetes mellitus. Am. J. Gastroenterol. 2001;96:1631–1634. & ) - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

