Adult bone marrow mesenchymal and neural crest stem cells are chemoattractive and accelerate motor recovery in a mouse model of spinal cord injury
- PMID: 26530515
- PMCID: PMC4632651
- DOI: 10.1186/s13287-015-0202-2
Adult bone marrow mesenchymal and neural crest stem cells are chemoattractive and accelerate motor recovery in a mouse model of spinal cord injury
Erratum in
-
Correction to: Adult bone marrow mesenchymal and neural crest stem cells are chemoattractive and accelerate motor recovery in a mouse model of spinal cord injury.Stem Cell Res Ther. 2021 Sep 22;12(1):509. doi: 10.1186/s13287-021-02534-z. Stem Cell Res Ther. 2021. PMID: 34551824 Free PMC article. No abstract available.
Abstract
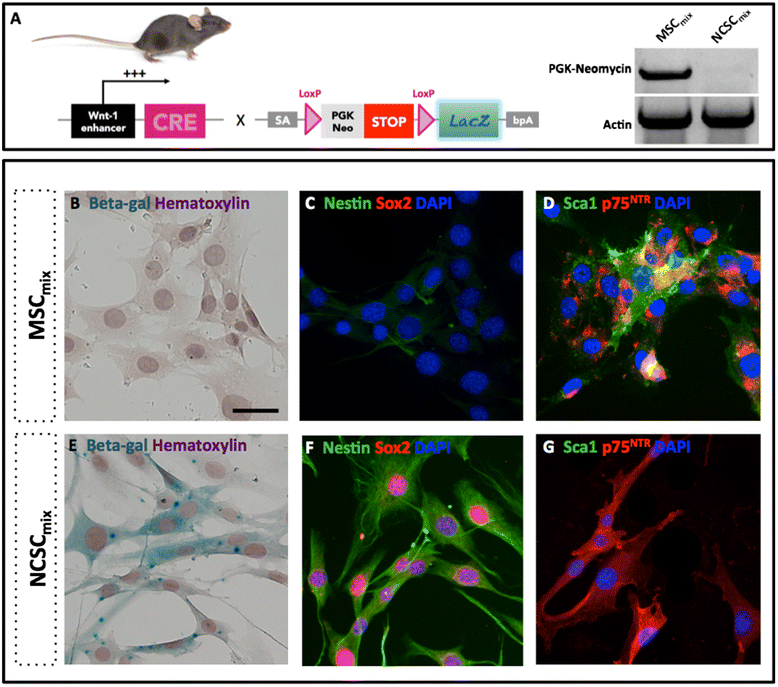
Introduction: Stem cells from adult tissues were considered for a long time as promising tools for regenerative therapy of neurological diseases, including spinal cord injuries (SCI). Indeed, mesenchymal (MSCs) and neural crest stem cells (NCSCs) together constitute the bone marrow stromal stem cells (BMSCs) that were used as therapeutic options in various models of experimental SCI. However, as clinical approaches remained disappointing, we thought that reducing BMSC heterogeneity should be a potential way to improve treatment efficiency and reproducibility.
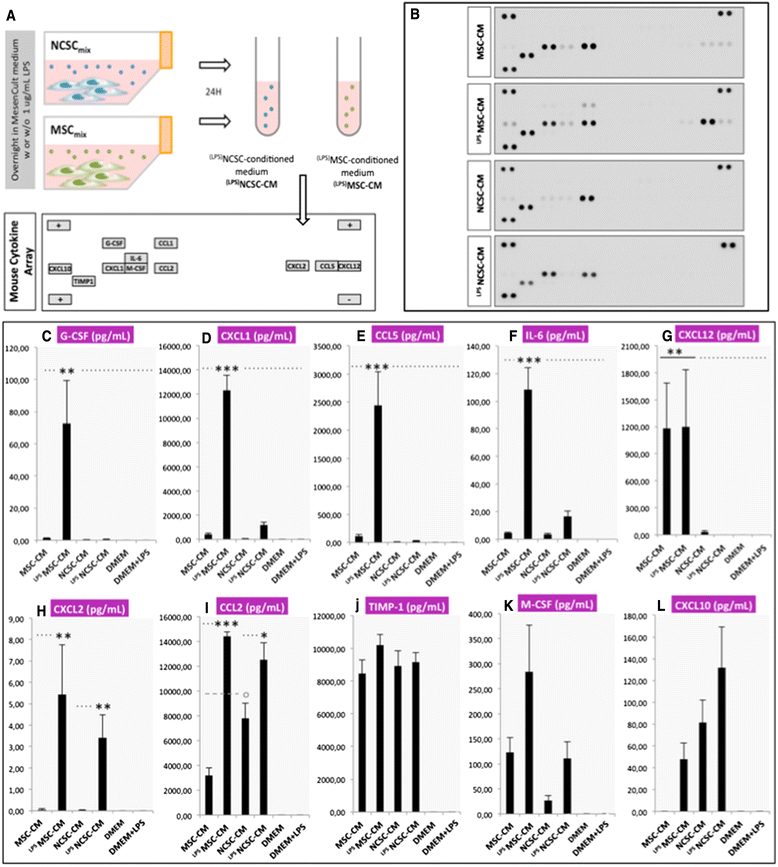
Methods: We investigated the impact of pure populations of MSCs and NCSCs isolated from adult bone marrow in a mouse model of spinal cord injury. We then analyzed the secretome of both MSCs and NCSCs, and its effect on macrophage migration in vitro.
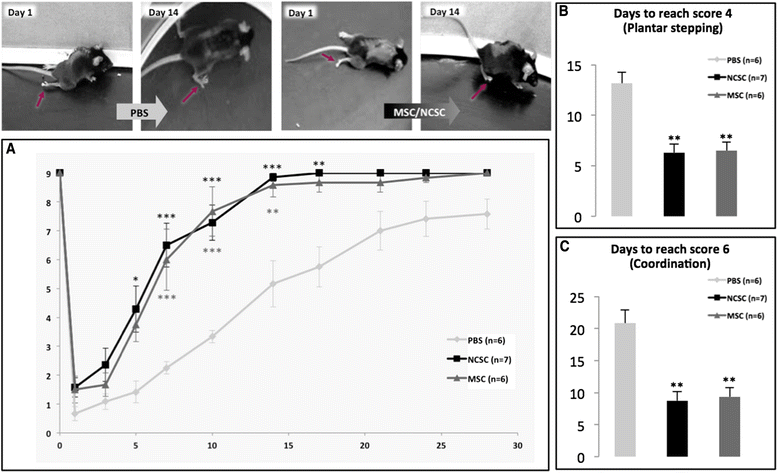
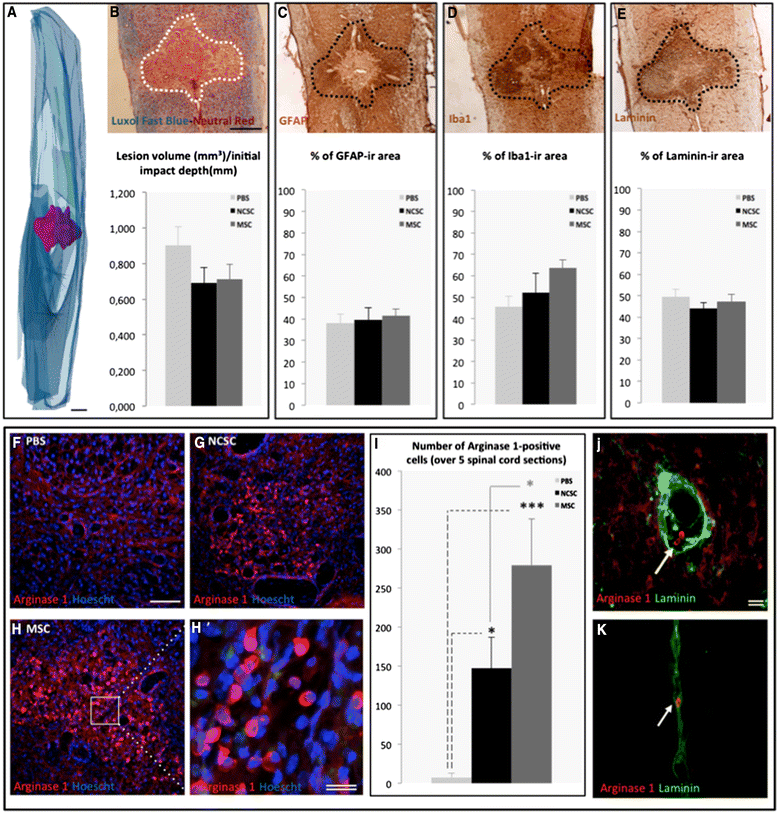
Results: We first observed that both cell types induced motor recovery in mice, and modified the inflammatory reaction in the lesion site. We also demonstrated that NCSCs but especially MSCs were able to secrete chemokines and attract macrophages in vitro. Finally, it appears that MSC injection in the spinal cord enhance early inflammatory events in the blood and spinal cord of SCI mice.
Conclusions: Altogether, our results suggest that both cell types have beneficial effects in experimental SCI, and that further investigation should be dedicated to the regulation of the inflammatory reaction following SCI, in the context of stem cell-based therapy but also in the early-phase clinical management of SCI patients.
Figures
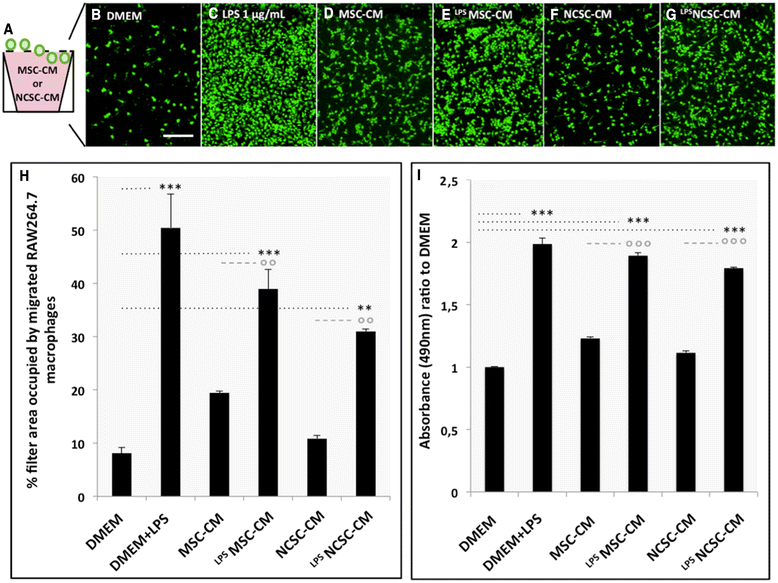
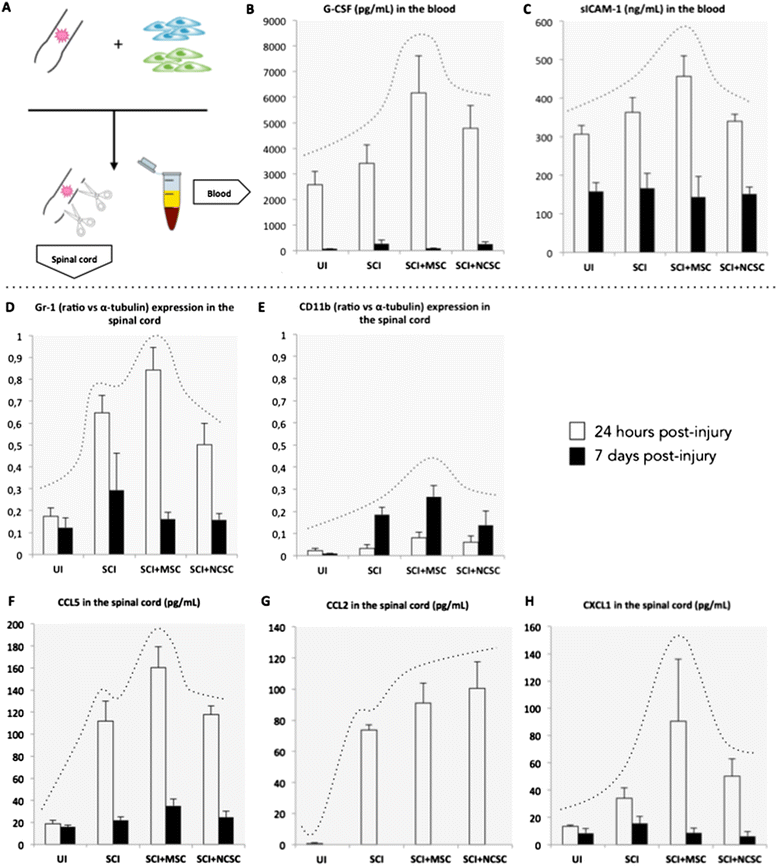
Comment in
-
Editor's Note: Adult bone marrow mesenchymal and neural crest stem cells are chemoattractive and accelerate motor recovery in a mouse model of spinal cord injury.Stem Cell Res Ther. 2021 Feb 15;12(1):135. doi: 10.1186/s13287-021-02197-w. Stem Cell Res Ther. 2021. PMID: 33588883 Free PMC article. No abstract available.
Similar articles
-
A combination of mesenchymal stem cells and scaffolds promotes motor functional recovery in spinal cord injury: a systematic review and meta-analysis.J Neurosurg Spine. 2019 Nov 1;32(2):269-284. doi: 10.3171/2019.8.SPINE19201. Print 2020 Feb 1. J Neurosurg Spine. 2019. PMID: 31675724
-
Bone marrow stem cells and polymer hydrogels--two strategies for spinal cord injury repair.Cell Mol Neurobiol. 2006 Oct-Nov;26(7-8):1113-29. doi: 10.1007/s10571-006-9007-2. Epub 2006 Apr 22. Cell Mol Neurobiol. 2006. PMID: 16633897 Review.
-
Treatment of rats with spinal cord injury using human bone marrow-derived stromal cells prepared by negative selection.J Biomed Sci. 2020 Feb 18;27(1):35. doi: 10.1186/s12929-020-00629-y. J Biomed Sci. 2020. PMID: 32066435 Free PMC article.
-
Neuronal regeneration in injured rat spinal cord after human dental pulp derived neural crest stem cell transplantation.Bratisl Lek Listy. 2018;119(3):143-151. doi: 10.4149/BLL_2018_028. Bratisl Lek Listy. 2018. PMID: 29536742
-
Bone marrow mesenchymal stem cells (BMSCs) improved functional recovery of spinal cord injury partly by promoting axonal regeneration.Neurochem Int. 2018 May;115:80-84. doi: 10.1016/j.neuint.2018.02.007. Epub 2018 Feb 16. Neurochem Int. 2018. PMID: 29458076 Review.
Cited by
-
Stem cell transplantation and functional recovery after spinal cord injury: a systematic review and meta-analysis.Anat Cell Biol. 2018 Sep;51(3):180-188. doi: 10.5115/acb.2018.51.3.180. Epub 2018 Sep 28. Anat Cell Biol. 2018. PMID: 30310710 Free PMC article.
-
Exosomes in perspective: a potential surrogate for stem cell therapy.Odontology. 2019 Jul;107(3):271-284. doi: 10.1007/s10266-018-0395-9. Epub 2018 Oct 15. Odontology. 2019. PMID: 30324571 Free PMC article. Review.
-
Mesenchymal stromal cell-derived secretome-based therapy for neurodegenerative diseases: overview of clinical trials.Stem Cell Res Ther. 2023 May 4;14(1):122. doi: 10.1186/s13287-023-03264-0. Stem Cell Res Ther. 2023. PMID: 37143147 Free PMC article. Review.
-
Correction to: Adult bone marrow mesenchymal and neural crest stem cells are chemoattractive and accelerate motor recovery in a mouse model of spinal cord injury.Stem Cell Res Ther. 2021 Sep 22;12(1):509. doi: 10.1186/s13287-021-02534-z. Stem Cell Res Ther. 2021. PMID: 34551824 Free PMC article. No abstract available.
-
Hypoxic preconditioning enhances the differentiation of bone marrow stromal cells into mature oligodendrocytes via the mTOR/HIF-1α/VEGF pathway in traumatic brain injury.Brain Behav. 2020 Jul;10(7):e01675. doi: 10.1002/brb3.1675. Epub 2020 May 30. Brain Behav. 2020. PMID: 32475084 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

