Identification of a novel platelet antagonist that binds to CLEC-2 and suppresses podoplanin-induced platelet aggregation and cancer metastasis
- PMID: 26528756
- PMCID: PMC4767466
- DOI: 10.18632/oncotarget.5811
Identification of a novel platelet antagonist that binds to CLEC-2 and suppresses podoplanin-induced platelet aggregation and cancer metastasis
Abstract
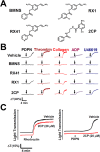
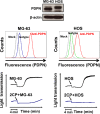
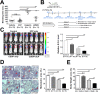
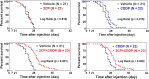
Podoplanin (PDPN) enhances tumor metastases by eliciting tumor cell-induced platelet aggregation (TCIPA) through activation of platelet C-type lectin-like receptor 2 (CLEC-2). A novel and non-cytotoxic 5-nitrobenzoate compound 2CP was synthesized that specifically inhibited the PDPN/CLEC-2 interaction and TCIPA with no effect on platelet aggregation stimulated by other platelet agonists. 2CP possessed anti-cancer metastatic activity in vivo and augmented the therapeutic efficacy of cisplatin in the experimental animal model without causing a bleeding risk. Analysis of the molecular action of 2CP further revealed that Akt1/PDK1 and PKCμ were two alternative CLEC-2 signaling pathways mediating PDPN-induced platelet activation. 2CP directly bound to CLEC-2 and, by competing with the same binding pocket of PDPN in CLEC-2, inhibited PDPN-mediated platelet activation. This study provides evidence that 2CP is the first defined platelet antagonist with CLEC-2 binding activity. The augmentation in the therapeutic efficacy of cisplatin by 2CP suggests that a combination of a chemotherapeutic agent and a drug with anti-TCIPA activity such as 2CP may prove clinically effective.
Keywords: TCIPA; platelet aggregation; podoplanin; tumor metastasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Functional characterization of recombinant snake venom rhodocytin: rhodocytin mutant blocks CLEC-2/podoplanin-dependent platelet aggregation and lung metastasis.J Thromb Haemost. 2018 May;16(5):960-972. doi: 10.1111/jth.13987. Epub 2018 Mar 30. J Thromb Haemost. 2018. PMID: 29488681
-
Targeting a novel domain in podoplanin for inhibiting platelet-mediated tumor metastasis.Oncotarget. 2016 Jan 26;7(4):3934-46. doi: 10.18632/oncotarget.6598. Oncotarget. 2016. PMID: 26684030 Free PMC article.
-
Polysaccharide-containing fraction from Artemisia argyi inhibits tumor cell-induced platelet aggregation by blocking interaction of podoplanin with C-type lectin-like receptor 2.J Food Drug Anal. 2020 Jan;28(1):115-123. doi: 10.1016/j.jfda.2019.08.002. Epub 2019 Sep 26. J Food Drug Anal. 2020. PMID: 31883599
-
[Identification of the novel platelet activation receptor CLEC-2 and Its pathological and physiological roles].Rinsho Byori. 2010 Dec;58(12):1193-202. Rinsho Byori. 2010. PMID: 21348239 Review. Japanese.
-
Platelet CLEC2-Podoplanin Axis as a Promising Target for Oral Cancer Treatment.Front Immunol. 2021 Dec 15;12:807600. doi: 10.3389/fimmu.2021.807600. eCollection 2021. Front Immunol. 2021. PMID: 34987523 Free PMC article. Review.
Cited by
-
A safety study of newly generated anti-podoplanin-neutralizing antibody in cynomolgus monkey (Macaca fascicularis).Oncotarget. 2018 Sep 7;9(70):33322-33336. doi: 10.18632/oncotarget.26055. eCollection 2018 Sep 7. Oncotarget. 2018. PMID: 30279963 Free PMC article.
-
Emerging roles of platelets in cancer biology and their potential as therapeutic targets.Front Oncol. 2022 Jul 22;12:939089. doi: 10.3389/fonc.2022.939089. eCollection 2022. Front Oncol. 2022. PMID: 35936717 Free PMC article. Review.
-
Podoplanin in Inflammation and Cancer.Int J Mol Sci. 2019 Feb 6;20(3):707. doi: 10.3390/ijms20030707. Int J Mol Sci. 2019. PMID: 30736372 Free PMC article. Review.
-
C-type lectin-like receptor 2: roles and drug target.Thromb J. 2024 Mar 19;22(1):27. doi: 10.1186/s12959-024-00594-8. Thromb J. 2024. PMID: 38504248 Free PMC article. Review.
-
Role of platelets and platelet receptors in cancer metastasis.J Hematol Oncol. 2018 Oct 11;11(1):125. doi: 10.1186/s13045-018-0669-2. J Hematol Oncol. 2018. PMID: 30305116 Free PMC article. Review.
References
-
- Stenger D, Dutting S, Nieswandt B. Mechanistic explanation for platelet contribution to cancer metastasis. Thromb Res. 2014;133:S149–157. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

