Assessing particle and fiber toxicology in the respiratory system: the stereology toolbox
- PMID: 26521139
- PMCID: PMC4628359
- DOI: 10.1186/s12989-015-0110-8
Assessing particle and fiber toxicology in the respiratory system: the stereology toolbox
Abstract
The inhalation of airborne particles can lead to pathological changes in the respiratory tract. For this reason, toxicology studies on effects of inhalable particles and fibers often include an assessment of histopathological alterations in the upper respiratory tract, the trachea and/or the lungs. Conventional pathological evaluations are usually performed by scoring histological lesions in order to obtain "quantitative" information and an estimation of the severity of the lesion. This approach not only comprises a potential subjective bias, depending on the examiner's judgment, but also conveys the risk that mild alterations escape the investigator's eye. The most accurate way of obtaining unbiased quantitative information about three-dimensional (3D) features of tissues, cells, or organelles from two-dimensional physical or optical sections is by means of stereology, the gold standard of image-based morphometry. Nevertheless, it can be challenging to express histopathological changes by morphometric parameters such as volume, surface, length or number only. In this review we therefore provide an overview on different histopathological lesions in the respiratory tract associated with particle and fiber toxicology and on how to apply stereological methods in order to correctly quantify and interpret histological lesions in the respiratory tract. The article further aims at pointing out common pitfalls in quantitative histopathology and at providing some suggestions on how respiratory toxicology can be improved by stereology. Thus, we hope that this article will stimulate scientists in particle and fiber toxicology research to implement stereological techniques in their studies, thereby promoting an unbiased 3D assessment of pathological lesions associated with particle exposure.
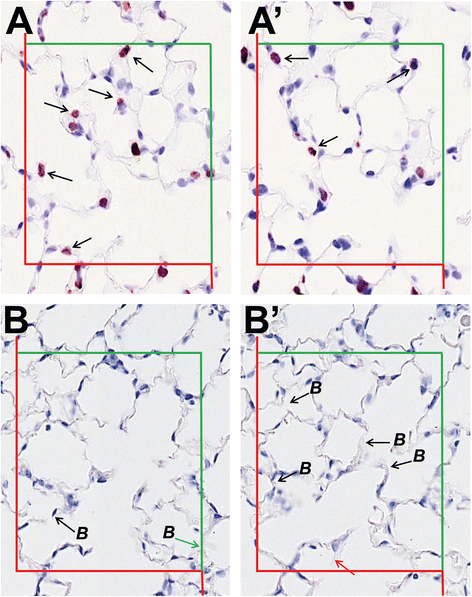
Figures
Similar articles
-
[Standard technical specifications for methacholine chloride (Methacholine) bronchial challenge test (2023)].Zhonghua Jie He He Hu Xi Za Zhi. 2024 Feb 12;47(2):101-119. doi: 10.3760/cma.j.cn112147-20231019-00247. Zhonghua Jie He He Hu Xi Za Zhi. 2024. PMID: 38309959 Chinese.
-
NTP Toxicity Study Report on the atmospheric characterization, particle size, chemical composition, and workplace exposure assessment of cellulose insulation (CELLULOSEINS).Toxic Rep Ser. 2006 Aug;(74):1-62, A1-C2. Toxic Rep Ser. 2006. PMID: 17160106
-
Stereology of the Peripheral Nervous System.Toxicol Pathol. 2020 Jan;48(1):37-48. doi: 10.1177/0192623319854746. Epub 2019 Jun 20. Toxicol Pathol. 2020. PMID: 31221020
-
Bias in image analysis and its solution: unbiased stereology.J Toxicol Pathol. 2017 Jul;30(3):183-191. doi: 10.1293/tox.2017-0013. Epub 2017 Mar 4. J Toxicol Pathol. 2017. PMID: 28798525 Free PMC article. Review.
-
A review of state-of-the-art stereology for better quantitative 3D morphology in cardiac research.Cardiovasc Pathol. 2010 Mar-Apr;19(2):65-82. doi: 10.1016/j.carpath.2008.10.015. Epub 2009 Jan 14. Cardiovasc Pathol. 2010. PMID: 19144544 Review.
Cited by
-
Using electron microscopes to look into the lung.Histochem Cell Biol. 2016 Dec;146(6):695-707. doi: 10.1007/s00418-016-1502-z. Epub 2016 Sep 29. Histochem Cell Biol. 2016. PMID: 27688057 Review.
-
miR-21-KO Alleviates Alveolar Structural Remodeling and Inflammatory Signaling in Acute Lung Injury.Int J Mol Sci. 2020 Jan 27;21(3):822. doi: 10.3390/ijms21030822. Int J Mol Sci. 2020. PMID: 32012801 Free PMC article.
-
Stereology as the 3D tool to quantitate lung architecture.Histochem Cell Biol. 2021 Feb;155(2):163-181. doi: 10.1007/s00418-020-01927-0. Epub 2020 Oct 13. Histochem Cell Biol. 2021. PMID: 33051774 Free PMC article. Review.
-
Air-blood barrier thickening and alterations of alveolar epithelial type 2 cells in mouse lungs with disrupted hepcidin/ferroportin regulatory system.Histochem Cell Biol. 2019 Mar;151(3):217-228. doi: 10.1007/s00418-018-1737-y. Epub 2018 Oct 3. Histochem Cell Biol. 2019. PMID: 30280242
-
Silica-Triggered Autoimmunity in Lupus-Prone Mice Blocked by Docosahexaenoic Acid Consumption.PLoS One. 2016 Aug 11;11(8):e0160622. doi: 10.1371/journal.pone.0160622. eCollection 2016. PLoS One. 2016. PMID: 27513935 Free PMC article.
References
-
- Hinkley GK, Roberts SM. Particle and Fiber Toxicology. In: Merkus HG, Meesters GMH, editors. Particulate Products: Tailoring Properties for Optimal Performance. Switzerland: Springer International Publishing AG; 2014. pp. 153–185.
-
- Maynard AD, Kuempel ED. Airborne nanostructured particles and occupational health. J Nanopart Res. 2005;7:587–614. doi: 10.1007/s11051-005-6770-9. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

