Role of mismatch repair proteins in the processing of cisplatin interstrand cross-links
- PMID: 26519826
- PMCID: PMC4651805
- DOI: 10.1016/j.dnarep.2015.10.003
Role of mismatch repair proteins in the processing of cisplatin interstrand cross-links
Abstract
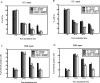
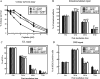
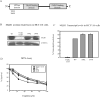
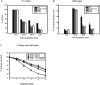
Mismatch repair (MMR) deficiency gives rise to cisplatin resistance and can lead to poor prognosis in cancers. Various models have been proposed to explain this low level of resistance caused due to loss of MMR proteins. We have shown that MMR proteins are required to maintain cisplatin interstrand cross-links (ICLs) on the DNA leading to increased cellular sensitivity. In our previous studies, we have shown that BER processing of the cisplatin ICLs is mutagenic. Polymerase β (Polβ) can generate mismatches which leads to the activation and the recruitment of mismatch repair proteins. In this paper, we distinguished between the requirement of different downstream MMR proteins for maintaining cisplatin sensitivity. We show that the MutSα (MSH2-MSH6) heterocomplex is required to maintain cisplatin sensitivity, whereas the Mutsβ complex has no effect. These results can be correlated with the increased repair of cisplatin ICLs and ICL induced DNA double strand breaks (DSBs) in the resistant cells. Moreover, we show that MLH1 proficient cells displayed a cisplatin sensitive phenotype when compared with the MLH1 deficient cells and the ATPase activity of MLH1 is essential to mediate this effect. Based on these results, we propose that MutSα as well as the downstream MMR pathway proteins are essential to maintain a cisplatin sensitive phenotype as a consequence of processing Polβ induced mismatches at sites flanking cisplatin ICLs.
Keywords: ATPase; BER; Cisplatin; MMR; Resistance.
Copyright © 2015 Elsevier B.V. All rights reserved.
Conflict of interest statement
None declared. RWS is a scientific consultant for Trevigen, Inc.
The authors’ state that there are no conflicts of. The work is not submitted to any other journal.
Figures

Similar articles
-
Epistatic role of base excision repair and mismatch repair pathways in mediating cisplatin cytotoxicity.Nucleic Acids Res. 2013 Aug;41(15):7332-43. doi: 10.1093/nar/gkt479. Epub 2013 Jun 12. Nucleic Acids Res. 2013. PMID: 23761438 Free PMC article.
-
Human MLH1 protein participates in genomic damage checkpoint signaling in response to DNA interstrand crosslinks, while MSH2 functions in DNA repair.PLoS Genet. 2008 Sep 12;4(9):e1000189. doi: 10.1371/journal.pgen.1000189. PLoS Genet. 2008. PMID: 18787700 Free PMC article.
-
Crosstalk between BRCA-Fanconi anemia and mismatch repair pathways prevents MSH2-dependent aberrant DNA damage responses.EMBO J. 2014 Aug 1;33(15):1698-712. doi: 10.15252/embj.201387530. Epub 2014 Jun 25. EMBO J. 2014. PMID: 24966277 Free PMC article.
-
Mismatch repair pathway: molecules, functions, and role in colorectal carcinogenesis.Eur J Cancer Prev. 2014 Jul;23(4):246-57. doi: 10.1097/CEJ.0000000000000019. Eur J Cancer Prev. 2014. PMID: 24614649 Review.
-
Signalling cell cycle arrest and cell death through the MMR System.Carcinogenesis. 2006 Apr;27(4):682-92. doi: 10.1093/carcin/bgi298. Epub 2005 Dec 6. Carcinogenesis. 2006. PMID: 16332722 Review.
Cited by
-
MLH1 enhances the sensitivity of human endometrial carcinoma cells to cisplatin by activating the MLH1/c-Abl apoptosis signaling pathway.BMC Cancer. 2018 Dec 29;18(1):1294. doi: 10.1186/s12885-018-5218-4. BMC Cancer. 2018. PMID: 30594176 Free PMC article.
-
HPV induction of APOBEC3 enzymes mediate overall survival and response to cisplatin in head and neck cancer.DNA Repair (Amst). 2020 Mar;87:102802. doi: 10.1016/j.dnarep.2020.102802. Epub 2020 Jan 16. DNA Repair (Amst). 2020. PMID: 31981740 Free PMC article.
-
Mechanism-based design of agents that selectively target drug-resistant glioma.Science. 2022 Jul 29;377(6605):502-511. doi: 10.1126/science.abn7570. Epub 2022 Jul 28. Science. 2022. PMID: 35901163 Free PMC article.
-
Clinical and Genome-Wide Analysis of Cisplatin-Induced Peripheral Neuropathy in Survivors of Adult-Onset Cancer.Clin Cancer Res. 2017 Oct 1;23(19):5757-5768. doi: 10.1158/1078-0432.CCR-16-3224. Epub 2017 Jun 13. Clin Cancer Res. 2017. PMID: 28611204 Free PMC article.
-
Anti-tumor Effects of Cisplatin Synergist in Combined Treatment with Clostridium novyi-NT Spores Against Hypoxic Microenvironments in a Mouse Model of Cervical Cancer Caused by TC-1 Cell Line.Adv Pharm Bull. 2023 Nov;13(4):817-826. doi: 10.34172/apb.2023.084. Epub 2023 May 20. Adv Pharm Bull. 2023. PMID: 38022809 Free PMC article.
References
-
- Iyer RR, Pluciennik A, Burdett V, Modrich PL. DNA mismatch repair: Functions and mechanisms. Chemical Reviews. 2006;106:302–323. - PubMed
-
- Fink D, Zheng H, Nebel S, Norris PS, Aebi S, Lin TP, Nehme A, Christen RD, Haas M, MacLeod CL, Howell SB. In vitro and in vivo resistance to cisplatin in cells that have lost DNA mismatch repair. Cancer Research. 1997;57:1841–1845. - PubMed
-
- Wang JYJ, Edelmann W. Mismatch repair proteins as sensors of alkylation DNA damage. Cancer Cell. 2006;9:417–418. - PubMed
-
- Pani E, Stojic L, El-Shemerly M, Jiricny J, Ferrari S. Mismatch repair status and the response of human cells to cisplatin. Cell Cycle. 2007;6:1796–1802. - PubMed
-
- Fink D, Nebel S, Aebi S, Zheng H, Cenni B, Nehme A, Christen RD, Howell SB. The role of DNA mismatch repair in platinum drug resistance. Cancer Research. 1996;56:4881–4886. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

