Co-transplantation of syngeneic mesenchymal stem cells improves survival of allogeneic glial-restricted precursors in mouse brain
- PMID: 26515691
- PMCID: PMC4688082
- DOI: 10.1016/j.expneurol.2015.10.008
Co-transplantation of syngeneic mesenchymal stem cells improves survival of allogeneic glial-restricted precursors in mouse brain
Abstract
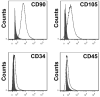
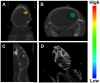
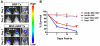
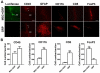
Loss of functional cells from immunorejection during the early post-transplantation period is an important factor that reduces the efficacy of stem cell-based therapies. Recent studies have shown that transplanted mesenchymal stem cells (MSCs) can exert therapeutic effects by secreting anti-inflammatory and pro-survival trophic factors. We investigated whether co-transplantation of MSCs could improve the survival of other transplanted therapeutic cells. Allogeneic glial-restricted precursors (GRPs) were isolated from the brain of a firefly luciferase transgenic FVB mouse (at E13.5 stage) and intracerebrally transplanted, either alone, or together with syngeneic MSCs in immunocompetent BALB/c mice (n=20) or immunodeficient Rag2(-/-) mice as survival control (n=8). No immunosuppressive drug was given to any animal. Using bioluminescence imaging (BLI) as a non-invasive readout of cell survival, we found that co-transplantation of MSCs significantly improved (p<0.05) engrafted GRP survival. No significant change in signal intensities was observed in immunodeficient Rag2(-/-) mice, with transplanted cells surviving in both the GRP only and the GRP+MSC group. In contrast, on day 21 post-transplantation, we observed a 94.2% decrease in BLI signal intensity in immunocompetent mice transplanted with GRPs alone versus 68.1% in immunocompetent mice co-transplanted with MSCs and GRPs (p<0.05). Immunohistochemical analysis demonstrated a lower number of infiltrating CD45, CD11b(+) and CD8(+) cells, reduced astrogliosis, and a higher number of FoxP3(+) cells at the site of transplantation for the immunocompetent mice receiving MSCs. The present study demonstrates that co-transplantation of MSCs can be used to create a microenvironment that is more conducive to the survival of allogeneic GRPs.
Keywords: Bioluminescence imaging; Cell survival; Co-transplantation; Glial-restricted precursor; Immunomodulation; Mesenchymal stem cell.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Syngeneic Mesenchymal Stem Cells Reduce Immune Rejection After Induced Pluripotent Stem Cell-Derived Allogeneic Cardiomyocyte Transplantation.Sci Rep. 2020 Mar 12;10(1):4593. doi: 10.1038/s41598-020-58126-z. Sci Rep. 2020. PMID: 32165680 Free PMC article.
-
Traumatic brain injury does not disrupt costimulatory blockade-induced immunological tolerance to glial-restricted progenitor allografts.J Neuroinflammation. 2021 Apr 30;18(1):104. doi: 10.1186/s12974-021-02152-9. J Neuroinflammation. 2021. PMID: 33931070 Free PMC article.
-
In Vivo Imaging of Allografted Glial-Restricted Progenitor Cell Survival and Hydrogel Scaffold Biodegradation.ACS Appl Mater Interfaces. 2021 May 26;13(20):23423-23437. doi: 10.1021/acsami.1c03415. Epub 2021 May 12. ACS Appl Mater Interfaces. 2021. PMID: 33978398 Free PMC article.
-
Mesenchymal stem cell therapy for neurodegenerative diseases.J Nanosci Nanotechnol. 2014 Jan;14(1):969-75. doi: 10.1166/jnn.2014.9126. J Nanosci Nanotechnol. 2014. PMID: 24730313 Review.
-
Stem cell delivery of therapies for brain disorders.Clin Transl Med. 2014 Jul 19;3:24. doi: 10.1186/2001-1326-3-24. eCollection 2014. Clin Transl Med. 2014. PMID: 25097727 Free PMC article. Review.
Cited by
-
Transplantation of Human Glial Progenitors to Immunodeficient Neonatal Mice with Amyotrophic Lateral Sclerosis (SOD1/rag2).Antioxidants (Basel). 2022 May 26;11(6):1050. doi: 10.3390/antiox11061050. Antioxidants (Basel). 2022. PMID: 35739947 Free PMC article.
-
Immunological Characteristics and Properties of Glial Restricted Progenitors of Mice, Canine Primary Culture Suspensions, and Human QSV40 Immortalized Cell Lines for Prospective Therapies of Neurodegenerative Disorders.Cell Transplant. 2019 Sep-Oct;28(9-10):1140-1154. doi: 10.1177/0963689719848355. Epub 2019 May 24. Cell Transplant. 2019. PMID: 31124369 Free PMC article.
-
Intra-arterial transplantation of stem cells in large animals as a minimally-invasive strategy for the treatment of disseminated neurodegeneration.Sci Rep. 2021 Mar 22;11(1):6581. doi: 10.1038/s41598-021-85820-3. Sci Rep. 2021. PMID: 33753789 Free PMC article.
-
Murine glial progenitor cells transplantation and synthetic PreImplantation Factor (sPIF) reduces inflammation and early motor impairment in ALS mice.Sci Rep. 2022 Mar 7;12(1):4016. doi: 10.1038/s41598-022-08064-9. Sci Rep. 2022. PMID: 35256767 Free PMC article.
-
Serial in vivo imaging of transplanted allogeneic neural stem cell survival in a mouse model of amyotrophic lateral sclerosis.Exp Neurol. 2017 Mar;289:96-102. doi: 10.1016/j.expneurol.2016.12.011. Epub 2016 Dec 28. Exp Neurol. 2017. PMID: 28038988 Free PMC article.
References
-
- Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. - PubMed
-
- Apostolides C, Sanford E, Hong M, Mendez I. Glial cell line-derived neurotrophic factor improves intrastriatal graft survival of stored dopaminergic cells. Neuroscience. 1998;83:363–372. - PubMed
-
- Bantubungi K, Blum D, Cuvelier L, Wislet-Gendebien S, Rogister B, Brouillet E, Schiffmann SN. Stem cell factor and mesenchymal and neural stem cell transplantation in a rat model of Huntington’s disease. Mol Cell Neurosci. 2008;37:454–470. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

