Relating Cortical Atrophy in Temporal Lobe Epilepsy with Graph Diffusion-Based Network Models
- PMID: 26513579
- PMCID: PMC4626097
- DOI: 10.1371/journal.pcbi.1004564
Relating Cortical Atrophy in Temporal Lobe Epilepsy with Graph Diffusion-Based Network Models
Abstract
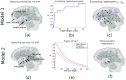
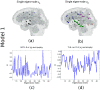
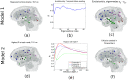
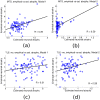
Mesial temporal lobe epilepsy (TLE) is characterized by stereotyped origination and spread pattern of epileptogenic activity, which is reflected in stereotyped topographic distribution of neuronal atrophy on magnetic resonance imaging (MRI). Both epileptogenic activity and atrophy spread appear to follow white matter connections. We model the networked spread of activity and atrophy in TLE from first principles via two simple first order network diffusion models. Atrophy distribution is modeled as a simple consequence of the propagation of epileptogenic activity in one model, and as a progressive degenerative process in the other. We show that the network models closely reproduce the regional volumetric gray matter atrophy distribution of two epilepsy cohorts: 29 TLE subjects with medial temporal sclerosis (TLE-MTS), and 50 TLE subjects with normal appearance on MRI (TLE-no). Statistical validation at the group level suggests high correlation with measured atrophy (R = 0.586 for TLE-MTS, R = 0.283 for TLE-no). We conclude that atrophy spread model out-performs the hyperactivity spread model. These results pave the way for future clinical application of the proposed model on individual patients, including estimating future spread of atrophy, identification of seizure onset zones and surgical planning.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Network Analysis on Predicting Mean Diffusivity Change at Group Level in Temporal Lobe Epilepsy.Brain Connect. 2016 Oct;6(8):607-620. doi: 10.1089/brain.2015.0381. Epub 2016 Sep 7. Brain Connect. 2016. PMID: 27405726 Free PMC article.
-
Structural and functional asymmetry of medial temporal subregions in unilateral temporal lobe epilepsy: A 7T MRI study.Hum Brain Mapp. 2019 Jun 1;40(8):2390-2398. doi: 10.1002/hbm.24530. Epub 2019 Jan 21. Hum Brain Mapp. 2019. PMID: 30666753 Free PMC article.
-
Hippocampal and thalamic atrophy in mild temporal lobe epilepsy: a VBM study.Neurology. 2008 Sep 30;71(14):1094-101. doi: 10.1212/01.wnl.0000326898.05099.04. Neurology. 2008. PMID: 18824674
-
Graph theory findings in the pathophysiology of temporal lobe epilepsy.Clin Neurophysiol. 2014 Jul;125(7):1295-305. doi: 10.1016/j.clinph.2014.04.004. Epub 2014 Apr 21. Clin Neurophysiol. 2014. PMID: 24831083 Free PMC article. Review.
-
MRI-negative temporal lobe epilepsy-What do we know?Epilepsia. 2017 May;58(5):727-742. doi: 10.1111/epi.13699. Epub 2017 Mar 7. Epilepsia. 2017. PMID: 28266710 Review.
Cited by
-
Neuroimaging and connectomics of drug-resistant epilepsy at multiple scales: From focal lesions to macroscale networks.Epilepsia. 2019 Apr;60(4):593-604. doi: 10.1111/epi.14688. Epub 2019 Mar 19. Epilepsia. 2019. PMID: 30889276 Free PMC article. Review.
-
Temporal lobe epilepsy affects spatial organization of entorhinal cortex connectivity.Epilepsy Behav. 2018 Nov;88:87-95. doi: 10.1016/j.yebeh.2018.06.038. Epub 2018 Sep 20. Epilepsy Behav. 2018. PMID: 30243111 Free PMC article.
-
An Integrative Approach to Study Structural and Functional Network Connectivity in Epilepsy Using Imaging and Signal Data.Front Integr Neurosci. 2021 Jan 12;14:491403. doi: 10.3389/fnint.2020.491403. eCollection 2020. Front Integr Neurosci. 2021. PMID: 33510622 Free PMC article.
-
Longitudinal increases in structural connectome segregation and functional connectome integration are associated with better recovery after mild TBI.Hum Brain Mapp. 2019 Oct 15;40(15):4441-4456. doi: 10.1002/hbm.24713. Epub 2019 Jul 11. Hum Brain Mapp. 2019. PMID: 31294921 Free PMC article.
-
Mode-based morphometry: A multiscale approach to mapping human neuroanatomy.Hum Brain Mapp. 2024 Mar;45(4):e26640. doi: 10.1002/hbm.26640. Hum Brain Mapp. 2024. PMID: 38445545 Free PMC article.
References
-
- Keller SS, Wieshmann UC, Mackay CE, Denby CE, Webb J, et al. (2002) Voxel based morphometry of grey matter abnormalities in patients with medically intractable temporal lobe epilepsy: effects of side of seizure onset and epilepsy duration. Journal of Neurology, Neurosurgery & Psychiatry 73: 648–655. 10.1136/jnnp.73.6.648 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

